Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death
- PMID: 18781889
- PMCID: PMC2694227
- DOI: 10.1089/neu.2008.0512
Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death
Abstract
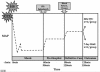
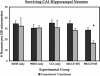
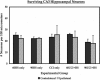
Traumatic brain injury (TBI) from blast injury is often complicated by hemorrhagic shock (HS) in victims of terrorist attacks. Most studies of HS after experimental TBI have focused on intracranial pressure; few have explored the effect of HS on neuronal death after TBI, and none have been done in mice. We hypothesized that neuronal death in CA1 hippocampus would be exacerbated by HS after experimental TBI. C57BL6J male mice were anesthetized with isoflurane, mean arterial blood pressure (MAP) was monitored, and controlled cortical impact (CCI) delivered to the left parietal cortex followed by continued anesthesia (CCI-only), or either 60 or 90 min of volume-controlled HS. Parallel 60- or 90-min HS-only groups were also studied. After HS (+/-CCI), 6% hetastarch was used targeting MAP of > or =50 mm Hg during a 30-min Pre-Hospital resuscitation phase. Then, shed blood was re-infused, and hetastarch was given targeting MAP of > or =60 mm Hg during a 30-min Definitive Care phase. Neurological injury was evaluated at 24 h (fluorojade C) or 7 days (CA1 and CA3 hippocampal neuron counts). HS reduced MAP to 30-40 mm Hg in all groups, p < 0.05 versus CCI-only. Ipsilateral CA1 neuron counts in the 90-min CCI+HS group were reduced at 16.5 +/- 14.1 versus 30.8 +/- 6.8, 32.3 +/- 7.6, 30.6 +/- 2.2, 28.1 +/- 2.2 neurons/100 mum in CCI-only, 60-min HS-only, 90-min HS-only, and 60-min CCI+HS, respectively, all p < 0.05. CA3 neuron counts did not differ between groups. Fluorojade C staining confirmed neurodegeneration in CA1 in the 90-min CCI+HS group. Our data suggest a critical time window for exacerbation of neuronal death by HS after CCI and may have implications for blast injury victims in austere environments where definitive management is delayed.
Figures


References
-
- Barron K.D. Dentinger M.P. Kimelberg H.K. Nelson L.R. Bourke R.S. Keegan S. Mankes R. Cragoe E.J., Jr. Ultrastructural features of a brain injury model in cat. I. Vascular and neuroglial changes and the prevention of astroglial swelling by fluorenyl (aryloxy) alkanoic acid derivative (L-664, 711) Acta Neuropapthol. 1988;75:295–307. - PubMed
-
- Barzó P. Marmarou A. Fatouros P. Corwin F. Dunbar J.G. Acute blood-brain barrier changes in experimental closed head injury as measured by MRI and Gd-DTPA. Acta Neurochir. Suppl. 1997;70:243–246. - PubMed
-
- Bedell E.A. DeWitt D.S. Prough D.S. Fentanyl infusion preserves cerebral blood flow during decreased arterial blood pressure after traumatic brain injury in cats. J. Neurotrauma. 1998;15:985–992. - PubMed
-
- Bryan R.M. Cherian L. Robertson C. Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 1995;80:687–695. - PubMed
-
- Carillo P. Takasu A. Safar P. Severe hemorrhagic shock and resuscitation in rats does not cause subtle brain damage. J. Trauma. 1998;45:239–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

