Influence of enzyme-inducing antiepileptic drugs on trough level of imatinib in glioblastoma patients
- PMID: 18781906
- PMCID: PMC2748699
- DOI: 10.2174/157488408785747656
Influence of enzyme-inducing antiepileptic drugs on trough level of imatinib in glioblastoma patients
Abstract
Background: Imatinib mesylate is used in combination with hydroxyurea (HU) in ongoing clinical phase II studies in recurrent glioblastoma multiforme (GBM). CYP3A4 enzyme-inducing antiepileptic drugs (EIAEDs) like carbamazepine, phenytoin, and oxcarbazepine--as well as non-EIAEDs like valproic acid, levetiracetam, and lamotrigine--are frequently used in patients with GBM. Since CYP3A4 is the major isozyme involved in the metabolism of imatinib, we investigated the influence of EIAEDs on imatinib pharmacokinetics (pk).
Methods: GBM patients received 600 mg imatinib p.o./o.d. in combination with 1.0 g HU p.o./o.d..together with either EIAEDs, non-EIAEDs, or no antiepileptic drug (non-AEDs) comedication. Trough plasma levels of imatinib and its active main metabolite N-desmethyl-imatinib (CGP74588) were determined biweekly in these patients, total 543 samples being collected from 224 patients (up to 6 times / patient). All three groups were compared to each other and with historical pharmacokinetic data obtained from patients with chronic myeloid leukemia (CML).
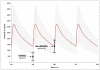
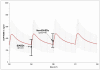
Results: Mean imatinib trough levels in patients not receiving AEDs ( 1404 ng/ml, CV 64%) and on non-EIAEDs (1374 ng/ml, CV 46%) were comparable with mean imatinib trough levels of the historical control group of CML patients (1400 ng/ml, CV 50%). Mean trough levels of imatinib were reduced up to 2.9-fold (477 ng/ml, CV 70%) in patients treated with EIAEDs. Only slight, but although significant differences were observed in the mean trough level of the metabolite CGP74588 between EIAED-, non-EIAED and no-AED patients, 240 ng/ml (CV 57%), 351 ng/ml (CV 34%) and 356 ng/ml (CV 52%), respectively. The corresponding mean level for CML patients was 300 ng/ml (CV 50%).
Conclusion: Significant decreases of imatinib and CGP74588 trough levels were observed for patients receiving EIAEDs. The EIAED-induced reduction in trough imatinib levels can be avoided by switching to non-EIAEDs comedication or compensated by administering higher imatinib doses. In addition these data demonstrate that there is no significant difference in the pharmacokinetics of imatinib between patients with glioblastoma and CML.
Figures
References
-
- Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. - PubMed
-
- Hou LC, Veeravagu A, Hsu AR, Tse VCL. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20:E5. - PubMed
-
- Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology and End Results (SEER) data 1973-1991. J NeuroSurg. 1998;88:1–10. - PubMed
-
- Dehdashti AR, Hegi ME, Regli L, Pica A, Stupp R. New trends in the medical management of glioblastoma multiforme: the role of temozolomide chemotherapy. Neurosurg Focus. 2006;20:E6. - PubMed
-
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
