Arabidopsis thaliana GLX2-1 contains a dinuclear metal binding site, but is not a glyoxalase 2
- PMID: 18782082
- PMCID: PMC2677751
- DOI: 10.1042/BJ20081151
Arabidopsis thaliana GLX2-1 contains a dinuclear metal binding site, but is not a glyoxalase 2
Abstract
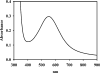
In an effort to probe the structure and function of a predicted mitochondrial glyoxalase 2, GLX2-1, from Arabidopsis thaliana, GLX2-1 was cloned, overexpressed, purified and characterized using metal analyses, kinetics, and UV-visible, EPR, and (1)H-NMR spectroscopies. The purified enzyme was purple and contained substoichiometric amounts of iron and zinc; however, metal-binding studies reveal that GLX2-1 can bind nearly two equivalents of either iron or zinc and that the most stable analogue of GLX2-1 is the iron-containing form. UV-visible spectra of the purified enzyme suggest the presence of Fe(II) in the protein, but the Fe(II) can be oxidized over time or by the addition of metal ions to the protein. EPR spectra revealed the presence of an anti-ferromagnetically-coupled Fe(III)Fe(II) centre and the presence of a protein-bound high-spin Fe(III) centre, perhaps as part of a FeZn centre. No paramagnetically shifted peaks were observed in (1)H-NMR spectra of the GLX2-1 analogues, suggesting low amounts of the paramagnetic, anti-ferromagnetically coupled centre. Steady-state kinetic studies with several thiolester substrates indicate that GLX2-1 is not a GLX2. In contrast with all of the other GLX2 proteins characterized, GLX2-1 contains an arginine in place of one of the metal-binding histidine residues at position 246. In order to evaluate further whether Arg(246) binds metal, the R246L mutant was prepared. The metal binding results are very similar to those of native GLX2-1, suggesting that a different amino acid is recruited as a metal-binding ligand. These results demonstrate that Arabidopsis GLX2-1 is a novel member of the metallo-beta-lactamase superfamily.
Figures



References
-
- Thornalley P. The glyoxalase system in health and disease. Mol. Aspects Med. 1993;14:287–371. - PubMed
-
- Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification – a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 1996;27:565–573. - PubMed
-
- Thornalley PJ. Methylglyoxal, glyoxalases and the development of diabetic complications. Amino Acids. 1994;6:15–23. - PubMed
-
- Thornalley PJ. Modification of the glyoxalase system in disease processes and prospects for therapeutic strategies. Biochem. Soc. Trans. 1993;21:531–534. - PubMed
-
- Tew KD. Is there a role for glyoxalase I inhibitors as antitumor drugs? Drug Resist. Updates. 2000;3:263–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

