Population pharmacokinetic and pharmacodynamic modelling of the effects of nicorandil in the treatment of acute heart failure
- PMID: 18782142
- PMCID: PMC2526243
- DOI: 10.1111/j.1365-2125.2008.03257.x
Population pharmacokinetic and pharmacodynamic modelling of the effects of nicorandil in the treatment of acute heart failure
Abstract
Aims: The aims of the study were 1) to evaluate the pharmacokinetics of nicorandil in healthy subjects and acute heart failure (AHF) patients and 2) to evaluate the exposure-response relationship with pulmonary arterial wedge pressure (PAWP) in AHF patients and to predict an appropriate dosing regimen for nicorandil.
Methods: Based on the data from two healthy volunteer and three AHF patient studies, models were developed to characterize the pharmacokinetics and pharmacodynamics of nicorandil. PAWP was used as the pharmacodynamic variable. An asymptotic exponential disease progression model was used to account for time dependent changes in PAWP that were not explained by nicorandil exposure. The modelling was performed using NONMEM version V.
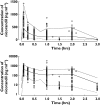
Results: The pharmacokinetics of nicorandil were characterized by a two-compartment model with linear elimination. CL, V1 and V2 in AHF patients were 1.96, 1.39 and 4.06 times greater than in healthy subjects. Predicted plasma concentrations were assumed to have an immediate concentration effect relationship on PAWP. An inhibitory E(max) model with E(max) of -11.7 mmHg and EC(50) of 423 microg l(-1) was considered the best relationship between nicorandil concentrations and PAWP. PAWP decreased independently of nicorandil exposure. This drug independent decline was described by an asymptotic decrease of 6.1 mmHg with a half-life of 5.3 h.
Conclusions: AHF patients have higher clearance and initial distribution volume of nicorandil compared with healthy subjects. The median target nicorandil concentration to decrease PAWP by 30% is predicted to be 748 microg l(-1), indicating that a loading dose of 200 microg kg(-1) and a maintenance dose of 400 microg kg(-1) h(-1) would be appropriate for the initial treatment of AHF.
Figures


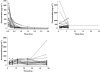
 ), median (
), median ( ), high (
), high ( ), observed (
), observed ( )
)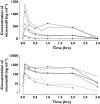
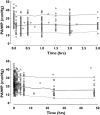
 ), median (
), median ( ), high (
), high ( ), OBS low (
), OBS low ( ), OBS median (
), OBS median ( ), OBS high (
), OBS high ( )
)
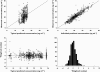
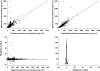
 ), median (
), median ( ), high (
), high ( ), observed (
), observed ( )
)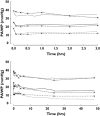
 ), median (
), median ( ), high (
), high ( ), OBS low (
), OBS low ( ), OBS median (
), OBS median ( ), OBS high (
), OBS high ( )
)References
-
- Nieminen MS, Bohm M, Cowie MR, Drexler H, Filippatos GS, Jondeau G, Hasin Y, Lopez-Sendon J, Mebazaa A, Metra M, Rhodes A, Swedberg K, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie MR, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, Morais J, Oto A, Smiseth OA, Garcia MA, Dickstein K, Albuquerque A, Conthe P, Crespo-Leiro M, Ferrari R, Follath F, Gavazzi A, Janssens U, Komajda M, Morais J, Moreno R, Singer M, Singh S, Tendera M, Thygesen K. Executive summary of the guidelines on the diagnosis and treatment of Acute Heart Failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:384–416. - PubMed
-
- Kamijo T, Kamei K, Sugo I, Kamiyama T, Sudo H, Ohba Y. Hemodynamic and hormonal responses to nicorandil in a canine model of acute ischemic heart failure: a comparison with cromakalim and nitroglycerin. J Cardiovasc Pharmacol. 1999;33:93–101. - PubMed
-
- Tsutamoto T, Kinoshita M, Hisanaga T, Maeda Y, Maeda K, Wada A, Fukai D, Yoshida S. Comparison of hemodynamic effects and plasma cyclic guanosine monophosphate of nicorandil and nitroglycerin in patients with congestive heart failure. Am J Cardiol. 1995;75:1162–5. - PubMed
-
- Larsen AI, Goransson L, Aarsland T, Tamby JF, Dickstein K. Comparison of the degree of hemodynamic tolerance during intravenous infusion of nitroglycerin versus nicorandil in patients with congestive heart failure. Am Heart J. 1997;134:435–41. - PubMed
-
- Frydman AM, Chapelle P, Diekmann H, Bruno R, Thebault JJ, Bouthier J, Caplain H, Ungethuem W, Galliard C, Le Liboux A, Renard A, Gaillot J. Pharmacokinetics of nicorandil. Am J Cardiol. 1989;63:25J–33J. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

