Generation of functionally mature dendritic cells from elutriated monocytes using polyinosinic : polycytidylic acid and soluble CD40 ligand for clinical application
- PMID: 18782327
- PMCID: PMC2633224
- DOI: 10.1111/j.1365-2249.2008.03757.x
Generation of functionally mature dendritic cells from elutriated monocytes using polyinosinic : polycytidylic acid and soluble CD40 ligand for clinical application
Abstract
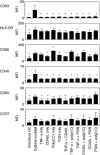
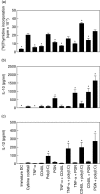
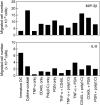
Despite the increasing use of dendritic cell (DC) vaccination in clinical trials, optimal conditions for the generation of functionally mature DCs remain to be established. The current standard DC maturation protocol for clinical trials has been used as an inflammatory cytokine cocktail [tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6 and prostaglandin E(2)], but this cocktail induced insufficient maturation of DCs derived from elutriated monocytes when cultured in X-VIVO 15. The aim of this study was to define effective combinations of stimulators for generating functionally mature DCs from elutriated monocytes under current good manufacturing practice conditions. We compared the functional capacity of DCs in response to all possible pairwise combinations of four different classes of stimuli: TNF-alpha, peptidoglycan, polyinosinic : polycytidylic acid [poly(I:C)] and soluble CD40 ligand (CD40L). Maturation status of DCs stimulated with combination of four stimuli was similar to that of the cytokine cocktail as assessed by the cell surface phenotype. However, only the combination of poly(I:C) + CD40L induced complete functional activation of the whole DC population, assessing IL-12p70 production, allostimulatory activity, migratory response to CCL19 and T helper 1-polarizing capacity. Thus, the protocol based on the combination of poly(I:C) and CD40L is more effective for the induction of clinical-grade DCs from elutriated monocytes than the standard cytokine cocktail.
Figures


References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2006;25:377–413. - PubMed
-
- Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Ann Rev Immunol. 2000;18:245–73. - PubMed
-
- O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

