Optimizing bioavailability of oral administration of small peptides through pharmacokinetic and pharmacodynamic parameters: the effect of water and timing of meal intake on oral delivery of Salmon Calcitonin
- PMID: 18782439
- PMCID: PMC2551583
- DOI: 10.1186/1472-6904-8-5
Optimizing bioavailability of oral administration of small peptides through pharmacokinetic and pharmacodynamic parameters: the effect of water and timing of meal intake on oral delivery of Salmon Calcitonin
Abstract
Background: To investigate the influence of water intake and dose timing on the pharmacokinetic and pharmacodynamic parameters of an oral formulation of salmon calcitonin (sCT).
Methods: The study was a randomized, partially-blind, placebo-controlled, single dose, exploratory crossover phase I study. 56 healthy postmenopausal women were randomly assigned to receive five treatments. The treatments comprised a combination of study medication (SMC021 (0.8 mg sCT + 200 mg 5-CNAC), SMC021 placebo, or 200 IU Miacalcic NS nasal spray), water volume given with the tablet (50 or 200 ml water), and time between dosing and meal (10, 30, or 60 minutes pre-meal). Plasma sCT levels and changes in the bone resorption (C-terminal telopeptide of collagen type I) was investigated. Trial regristration.
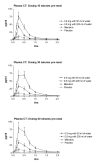
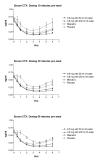
Results: Oral delivery of 0.8 mg of sCT with 50 ml of water compared to that with 200 ml water resulted in a two-fold increase in maximum concentration (Cmax and AUC0-4) of plasma sCT but comparable time to reach maximum concentration (Tmax). The sCT AUC0-4 with 50 ml of water was 4-fold higher than that obtained with nasal calcitonin. The increased absorption of sCT resulted in increased efficacy demonstrated by AUC of the relative change of serum CTX-I measured in the 6 hours post dosing.
Conclusion: 0.8 mg sCT with 50 ml of water taken 30 and 60 minutes prior to meal time resulted in optimal pharmacodynamic and pharmacokinetic parameters. The data suggest that this novel oral formulation may have improved absorption and reduction of bone resorption compared to that of the nasal form.
Figures
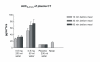
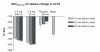
Similar articles
-
Influence of food intake on the bioavailability and efficacy of oral calcitonin.Br J Clin Pharmacol. 2009 Apr;67(4):413-20. doi: 10.1111/j.1365-2125.2009.03371.x. Br J Clin Pharmacol. 2009. PMID: 19371314 Free PMC article. Clinical Trial.
-
Investigations of inter- and intraindividual relationships between exposure to oral salmon calcitonin and a surrogate marker of pharmacodynamic efficacy.Eur J Clin Pharmacol. 2010 Jan;66(1):29-37. doi: 10.1007/s00228-009-0735-3. Epub 2009 Oct 8. Eur J Clin Pharmacol. 2010. PMID: 19813008 Clinical Trial.
-
The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study.Osteoarthritis Cartilage. 2010 Feb;18(2):150-9. doi: 10.1016/j.joca.2009.08.004. Epub 2009 Sep 1. Osteoarthritis Cartilage. 2010. PMID: 19747581 Clinical Trial.
-
Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials.J Clin Pharmacol. 2011 Apr;51(4):460-71. doi: 10.1177/0091270010372625. Epub 2010 Jul 21. J Clin Pharmacol. 2011. PMID: 20660294 Review.
-
Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis.Expert Opin Drug Metab Toxicol. 2016 Jun;12(6):681-9. doi: 10.1080/17425255.2016.1175436. Epub 2016 Apr 18. Expert Opin Drug Metab Toxicol. 2016. PMID: 27070719 Review.
Cited by
-
Calcitonin and Bone Physiology: In Vitro, In Vivo, and Clinical Investigations.Int J Endocrinol. 2020 Sep 10;2020:3236828. doi: 10.1155/2020/3236828. eCollection 2020. Int J Endocrinol. 2020. PMID: 32963524 Free PMC article. Review.
-
Absorption enhancers: applications and advances.AAPS J. 2012 Mar;14(1):10-8. doi: 10.1208/s12248-011-9307-4. Epub 2011 Nov 22. AAPS J. 2012. PMID: 22105442 Free PMC article. Review.
-
Biochemical markers identify influences on bone and cartilage degradation in osteoarthritis--the effect of sex, Kellgren-Lawrence (KL) score, body mass index (BMI), oral salmon calcitonin (sCT) treatment and diurnal variation.BMC Musculoskelet Disord. 2010 Jun 17;11:125. doi: 10.1186/1471-2474-11-125. BMC Musculoskelet Disord. 2010. PMID: 20565725 Free PMC article. Clinical Trial.
-
Effect of Various Dosing Conditions on the Pharmacokinetics of Oral Semaglutide, a Human Glucagon-Like Peptide-1 Analogue in a Tablet Formulation.Diabetes Ther. 2021 Jul;12(7):1915-1927. doi: 10.1007/s13300-021-01078-y. Epub 2021 Jun 2. Diabetes Ther. 2021. PMID: 34080123 Free PMC article.
-
The Effect of Food Intake on the Pharmacokinetics of Oral Basal Insulin: A Randomised Crossover Trial in Healthy Male Subjects.Clin Pharmacokinet. 2019 Nov;58(11):1497-1504. doi: 10.1007/s40262-019-00772-2. Clin Pharmacokinet. 2019. PMID: 31093929 Free PMC article. Clinical Trial.
References
-
- Sexton PM, Findlay DM, Martin TJ. Calcitonin. Curr Med Chem. 1999;6:1067–1093. - PubMed
-
- Deftos LJ. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6. Chapter 18. 2006. Calcitonin; pp. 115–117.
-
- Copp DH, Cameron EC, Cheney BA, Davidson AG, Henze KG. Evidence for calcitonin – a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 1962;70:638–49. 638–649. - PubMed
-
- Kumar MA, Foster GV, MacIntyre I. Further evidence for calcitonin. a rapid-acting hormone which lowers plasma-calcium. Lancet. 1963;2:480–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

