Efficacy, safety and tolerability of artesunate-mefloquine in the treatment of uncomplicated Plasmodium falciparum malaria in four geographic zones of Nigeria
- PMID: 18782445
- PMCID: PMC2542389
- DOI: 10.1186/1475-2875-7-172
Efficacy, safety and tolerability of artesunate-mefloquine in the treatment of uncomplicated Plasmodium falciparum malaria in four geographic zones of Nigeria
Abstract
Background: The combination of artesunate and mefloquine has been reported to be effective against multi-drug resistant Plasmodium falciparum malaria, which has been reported in Nigeria. The objective of this multi-centre study was to evaluate the efficacy, safety and tolerability of the co-packaged formulation of artesunate and mefloquine in the treatment of uncomplicated malaria in two weight groups: those between 15 - 29 kg and > or = 30 kg respectively.
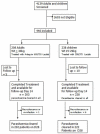
Methods: The trial was conducted in rural communities in the north-east, north-central, south-west and south-eastern parts of Nigeria. The WHO protocol for testing antimalarial drugs was followed. Outpatients having amongst other criteria, parasite density of > or =1,000 microl were enrolled. The co-packaged drugs were administered for 3 days at a dosage of artesunate, 4 mg/kg body wt/day and mefloquine, 25 mg/kg/body wt total) on days 0, 1 and 2. Patients were followed up for 28 days with the assessment of the parasitological parameters on days 1, 2, 3, 7, and 28.
Results: Four hundred and forty-six (446) patients were enrolled and 431 completed the study. Cure rates in both treatment groups was >90% at day 28. The mean parasite clearance times in treatment groups I and II were 40.1 and 42.4 hours respectively. The combination of artesunate and mefloquine showed good gametocidal activity, (gametocyte clearance time of 42.0 & 45.6 hours in treatment groups I and II respectively). There were no serious adverse events. Other adverse events observed were headache, dizziness, vomiting and abdominal discomfort. There was no significant derangement in the haematological and biochemical parameters.
Conclusion: This co-packaged formulation of artesunate + mefloquine (Artequin) is highly efficacious, safe and well-tolerated. It is recommended for the treatment of uncomplicated P. falciparum malaria in Nigeria.
Figures
References
-
- Teklehaimanot A, Bosman A. Opportunities, problems and prospects for malaria control in sub-Saharan Africa. Parassitologia. 1998;41:335–338. - PubMed
-
- National Antimalarial Policy Federal Ministry of Health, 2004, Abuja World Health Organization: The implementation of the global malaria control strategy: WHO Technical report series No 839, 1993, Geneva
-
- White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical