Analysis of polarization in QM/MM modelling of biologically relevant hydrogen bonds
- PMID: 18782723
- PMCID: PMC2585273
- DOI: 10.1098/rsif.2008.0243.focus
Analysis of polarization in QM/MM modelling of biologically relevant hydrogen bonds
Abstract
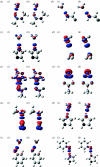
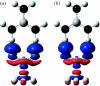
Combined quantum mechanics/molecular mechanics (QM/MM) methods are increasingly important for the study of chemical reactions and systems in condensed phases. Here, we have tested the accuracy of a density functional theory-based QM/MM implementation (B3LYP/6-311+G(d,p)/CHARMM27) on a set of biologically relevant interactions by comparison with full QM calculations. Intermolecular charge transfer due to hydrogen bond formation is studied to assess the severity of spurious polarization of QM atoms by MM point charges close to the QM/MM boundary. The changes in total electron density and natural bond orbital atomic charges due to hydrogen bond formation in selected complexes obtained at the QM/MM level are compared with full QM results. It is found that charge leakage from the QM atoms to MM atomic point charges close to the QM/MM boundary is not a serious problem, at least with limited basis sets. The results are encouraging in showing that important properties of key biomolecular interactions can be treated well at the QM/MM level employing good-quality levels of QM theory.
Figures
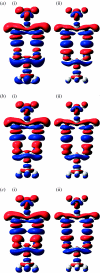


References
-
- Antes I., Thiel W. Adjusted connection atoms for combined quantum mechanical and molecular mechanical methods. J. Phys. Chem. A. 1999;103:9290–9295. doi: 10.1021/jp991771w. - DOI
-
- Aqvist J., Warshel A. Simulation of enzyme-reactions using valence-bond force-fields and other hybrid quantum-classical approaches. Chem. Rev. 1993;93:2523–2544. doi: 10.1021/cr00023a010. - DOI
-
- Assfeld X., Rivail J.L. Quantum chemical computations on parts of large molecules: the ab-initio local self consistent field method. Chem. Phys. Lett. 1996;263:100–106. doi: 10.1016/S0009-2614(96)01165-7. - DOI
-
- Bakowies D., Thiel W. Hybrid models for combined quantum mechanical and molecular mechanical approaches. J. Phys. Chem. A. 1996;100:10 580–10 594. doi: 10.1021/jp9536514. - DOI
-
- Bartha F., Kapuy O., Kozmutza C., Van Alsenoy C. Analysis of weakly bound structures: hydrogen bond and the electron density in a water dimer. J. Mol. Struct. (Theochem.) 2003;666:117–122. doi: 10.1016/j.theochem.2003.08.020. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

