MKP1 regulates the induction of MUC5AC mucin by Streptococcus pneumoniae pneumolysin by inhibiting the PAK4-JNK signaling pathway
- PMID: 18782768
- PMCID: PMC2576563
- DOI: 10.1074/jbc.M802519200
MKP1 regulates the induction of MUC5AC mucin by Streptococcus pneumoniae pneumolysin by inhibiting the PAK4-JNK signaling pathway
Abstract
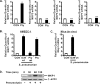
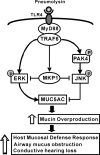
Mucosal epithelial cells in the respiratory tract act as the first line of host innate defense against inhaled microbes by producing a range of molecules for clearance. In particular, epithelial mucins facilitate the mucociliary clearance by physically trapping the inhaled microbes. Up-regulation of mucin production thus represents an important host innate defense response against invading microbes. Excess mucin production, however, overwhelms the mucociliary clearance, resulting in defective mucosal defenses. Thus, tight regulation of mucin production is critical for maintaining an appropriate balance between beneficial and detrimental outcomes. Among various mechanisms, negative regulation plays an important role in tightly regulating mucin production. Here we show that the PAK4-JNK signaling pathway acted as a negative regulator for Streptococcus pneumoniae pneumolysin-induced MUC5AC mucin transcription. Moreover pneumolysin also selectively induced expression of MKP1 via a TLR4-dependent MyD88-TRAF6-ERK signaling pathway, which inhibited the PAK4-JNK signaling pathway, thereby leading to up-regulation of MUC5AC mucin production to maintain effective mucosal protection against S. pneumoniae infection. These studies provide novel insights into the molecular mechanisms underlying the tight regulation of mucin overproduction in the pathogenesis of airway infectious diseases and may lead to development of new therapeutic strategies.
Figures


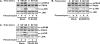


Similar articles
-
Vinpocetine inhibits Streptococcus pneumoniae-induced upregulation of mucin MUC5AC expression via induction of MKP-1 phosphatase in the pathogenesis of otitis media.J Immunol. 2015 Jun 15;194(12):5990-8. doi: 10.4049/jimmunol.1401489. Epub 2015 May 13. J Immunol. 2015. PMID: 25972475 Free PMC article.
-
Phosphodiesterase 4B mediates extracellular signal-regulated kinase-dependent up-regulation of mucin MUC5AC protein by Streptococcus pneumoniae by inhibiting cAMP-protein kinase A-dependent MKP-1 phosphatase pathway.J Biol Chem. 2012 Jun 29;287(27):22799-811. doi: 10.1074/jbc.M111.337378. Epub 2012 May 18. J Biol Chem. 2012. PMID: 22610099 Free PMC article.
-
A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae.J Immunol. 2007 Feb 1;178(3):1736-47. doi: 10.4049/jimmunol.178.3.1736. J Immunol. 2007. PMID: 17237423
-
Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production.Life Sci. 2019 Aug 15;231:116485. doi: 10.1016/j.lfs.2019.05.041. Epub 2019 May 19. Life Sci. 2019. PMID: 31116959 Review.
-
Toll-like receptor-dependent discrimination of streptococci.J Endotoxin Res. 2006;12(5):307-12. doi: 10.1179/096805106X118762. J Endotoxin Res. 2006. PMID: 17059694 Review.
Cited by
-
Role of Toll-like receptors 2 and 4 in pulmonary inflammation and injury induced by pneumolysin in mice.PLoS One. 2009 Nov 24;4(11):e7993. doi: 10.1371/journal.pone.0007993. PLoS One. 2009. PMID: 19956717 Free PMC article.
-
Home Dust Mites Promote MUC5AC Hyper-Expression by Modulating the sNASP/TRAF6 Axis in the Airway Epithelium.Int J Mol Sci. 2022 Aug 20;23(16):9405. doi: 10.3390/ijms23169405. Int J Mol Sci. 2022. PMID: 36012669 Free PMC article.
-
Vinpocetine inhibits Streptococcus pneumoniae-induced upregulation of mucin MUC5AC expression via induction of MKP-1 phosphatase in the pathogenesis of otitis media.J Immunol. 2015 Jun 15;194(12):5990-8. doi: 10.4049/jimmunol.1401489. Epub 2015 May 13. J Immunol. 2015. PMID: 25972475 Free PMC article.
-
Valsartan attenuates LPS-induced ALI by modulating NF-κB and MAPK pathways.Front Pharmacol. 2024 Jan 15;15:1321095. doi: 10.3389/fphar.2024.1321095. eCollection 2024. Front Pharmacol. 2024. PMID: 38288441 Free PMC article.
-
Lipidome and transcriptome profiling of pneumolysin intoxication identifies networks involved in statin-conferred protection of airway epithelial cells.Sci Rep. 2015 May 29;5:10624. doi: 10.1038/srep10624. Sci Rep. 2015. PMID: 26023727 Free PMC article.
References
-
- Kopp, E., and Medzhitov, R. (2003) Curr. Opin. Immunol. 15 396-401 - PubMed
-
- Rose, M. C., Nickola, T. J., and Voynow, J. A. (2001) Am. J. Respir. Cell Mol. Biol. 25 533-537 - PubMed
-
- Majima, Y., Hamaguchi, Y., Hirata, K., Takeuchi, K., Morishita, A., and Sakakura, Y. (1988) Ann. Otol. Rhinol. Laryngol. 97 272-274 - PubMed
-
- Klugman, K. P., and Feldman, C. (2001) Curr. Opin. Infect. Dis. 14 173-179 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

