TRPing on the lung endothelium: calcium channels that regulate barrier function
- PMID: 18783312
- PMCID: PMC2850299
- DOI: 10.1089/ars.2008.2221
TRPing on the lung endothelium: calcium channels that regulate barrier function
Abstract
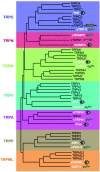
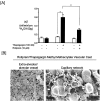
Rises in cytosolic calcium are sufficient to initiate the retraction of endothelial cell borders and to increase macromolecular permeability. Although endothelial cell biologists have recognized the importance of shifts in cytosolic calcium for several decades, only recently have we gained a rudimentary understanding of the membrane calcium channels that change cell shape. Members of the transient receptor potential family (TRP) are chief among the molecular candidates for permeability-coupled calcium channels. Activation of calcium entry through store-operated calcium entry channels, most notably TRPC1 and TRPC4, increases lung endothelial cell permeability, as does activation of calcium entry through the TRPV4 channel. However, TRPC1 and TRPC4 channels appear to influence the lung extraalveolar endothelial barrier most prominently, whereas TRPV4 channels appear to influence the lung capillary endothelial barrier most prominently. Thus, phenotypic heterogeneity in ion channel expression and function exists within the lung endothelium, along the arterial-capillary-venous axis, and is coupled to discrete control of endothelial barrier function.
Figures
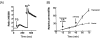




References
-
- Anderson JP. Morrow JS. The interaction of calmodulin with human erythrocyte spectrin. Inhibition of protein 4.1-stimulated actin binding. J Biol Chem. 1987;262:6365–6372. - PubMed
-
- Antoniotti S. Fiorio Pla A. Barral S. Scalabrino O. Munaron L. Lovisolo D. Interaction between TRPC channel subunits in endothelial cells. J Recept Signal Transduct Res. 2006;26:225–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

