Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions
- PMID: 18783346
- PMCID: PMC3936483
- DOI: 10.1042/BJ20081240
Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions
Abstract
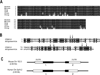
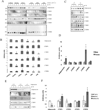
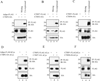
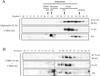
The insulin-sensitizing hormone, adiponectin, belongs to the expanding C1q/TNF (tumour necrosis factor) family of proteins. We recently identified a family of adiponectin paralogues designated as CTRP (C1q/TNF-related protein) 1-7, and in the present study describe CTRP10. In the present study, we show that CTRP1, CTRP2, CTRP3, CTRP5 and CTRP7 transcripts are expressed predominantly by adipose tissue. In contrast, placenta and eye expressed the highest levels of CTRP6 and CTRP10 transcripts respectively. Expression levels of CTRP1, CTRP2, CTRP3, CTRP6 and CTRP7 transcripts are up-regulated in 8-week-old obese (ob/ob) mice relative to lean controls. Treatment of mice with a PPAR-gamma (peroxisome-proliferator-activated receptor-gamma) agonist, rosiglitazone, increased the expression of CTRP1 and decreased CTRP6 transcript levels. All CTRPs are secreted glycoproteins when expressed in mammalian cells. CTRP1, CTRP2, CTRP3, CTRP5 and CTRP6 circulate in the blood and are potential endocrine hormones; their serum levels vary according to the sex and genetic background of mice. Importantly, serum levels of CTRP1 and CTRP6 are increased in adiponectin-null mice. Like adiponectin, all secreted CTRP proteins form trimers as their basic structural units. CTRP3, CTRP5, CTRP6 and CTRP10 trimers are further assembled into higher-order oligomeric complexes via disulfide bonding mediated by their N-terminal cysteine residues. Besides forming homo-oligomers, CTRP1/CTRP6, CTRP2/CTRP7 and adiponectin/CTRP2 are secreted as heterotrimers, thus providing a mechanism to potentially generate functionally distinct ligands. Functional characterization of one such family member, CTRP1, showed that it specifically activates Akt and p44/42-MAPK (mitogen-activated protein kinase) signalling pathways in differentiated mouse myotubes. Moreover, injection of recombinant CTRP1 into mice significantly reduced their serum glucose levels. Thus at least CTRP1 may be considered a novel adipokine. In summary, these molecular, biochemical and functional data provide an important framework to further address the physiological functions and mechanisms of the action of this family of secreted glycoproteins in normal and disease states.
Figures







Comment in
-
Adiponectin: no longer the lone soul in the fight against insulin resistance?Biochem J. 2008 Dec 1;416(2):e7-9. doi: 10.1042/BJ20082033. Biochem J. 2008. PMID: 18990088
References
-
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocrin. Rev. 2006;27:762–778. - PubMed
-
- Ahima RS, Qi Y, Singhal NS, Jackson MB, Scherer PE. Brain adipocytokine action and metabolic regulation. Diabetes. 2006;55(Suppl. 2):S145–S154. - PubMed
-
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. - PubMed
-
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. - PubMed
-
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) . Biochem. Biophys. Res. Commun. 1996;221:286–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

