Psychological mediators of bupropion sustained-release treatment for smoking cessation
- PMID: 18783504
- PMCID: PMC2879164
- DOI: 10.1111/j.1360-0443.2008.02275.x
Psychological mediators of bupropion sustained-release treatment for smoking cessation
Abstract
Aim: The study aimed to test simultaneously our understanding of the effects of bupropion sustained-release (SR) treatment on putative mediators and our understanding of determinants of post-quit abstinence, including withdrawal distress, cigarette craving, positive affect and subjective reactions to cigarettes smoked during a lapse. The specificity of bupropion SR effects was also tested in exploratory analyses.
Design: Data from a randomized, placebo-controlled clinical trial of bupropion SR were submitted to mediation analyses.
Setting: Center for Tobacco Research and Intervention, Madison, WI, USA.
Participants: A total of 403 adult, daily smokers without contraindications to bupropion SR use.
Intervention: Participants were assigned randomly to receive a 9-week course of bupropion SR or placebo pill and to receive eight brief individual counseling sessions or no counseling.
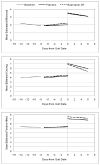
Measurements: Ecological momentary assessment ratings of smoking behavior and putative mediators were collected pre- and post-quit.
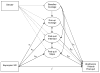
Findings: Results of structural equation and hierarchical linear models did not support the hypothesis that bupropion SR treatment improves short-term abstinence by reducing withdrawal distress or affecting the subjective effects of a lapse cigarette, but provided partial support for mediation by cigarette craving reduction and enhanced positive affect. Bupropion SR effects on point-prevalence abstinence at 1 month post-quit were also mediated partially by enhanced motivation to quit and self-efficacy.
Conclusions: Results provided some support for models of bupropion SR treatment and relapse and suggested that motivational processes may partially account for bupropion SR efficacy.
Conflict of interest statement
Conflict of Interest Statement
Douglas E. Jorenby has received research support from Nabi Biopharmaceutical and Pfizer, Inc. and consulting fees from Nabi Biopharmaceutical. Saul Shiffman serves as consultant to GlaxoSmithKline Consumer Healthcare on an exclusive basis regarding OTC smoking cessation products and also is a partner in a company that is developing a new nicotine medication. He is a co-founder of invivodata, inc., which provides electronic diary services for clinical research. Timothy B. Baker has served as a consultant, given lectures sponsored by, or has conducted research sponsored by GlaxoSmithKline, Nabi Biopharmaceuticals, Pfizer, and Sanofi-Synthelabo.
Figures
References
-
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Clinical Practice Guideline. Rockville (MD): U.S. Department of Health and Human Services. Public Health Service; Jun, 2000. Treating Tobacco Dependence and Use.
-
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2004;(4) - PubMed
-
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–20. - PubMed
-
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10:219–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

