Chemoenzymatic synthesis of N-linked neoglycoproteins through a chitinase-catalyzed transglycosylation
- PMID: 18783954
- PMCID: PMC2614311
- DOI: 10.1016/j.bmc.2008.08.042
Chemoenzymatic synthesis of N-linked neoglycoproteins through a chitinase-catalyzed transglycosylation
Abstract
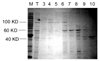
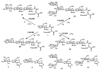
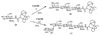
A novel application of the Bacillus sp. chitinase for the chemoenzymatic synthesis of N-linked neoglycoproteins is described. Three chitinases with different molecular size were purified from the crude chitinase preparation. The purified chitinases were evaluated for their hydrolytic and transglycosylation activity. One chitinase with a molecular size of 100 kDa (Chi100) was identified to be the one with highest transglycosylation/hydrolysis ratio. Chi100 could effectively recognize LacNAc-oxazoline and Manalpha1,3Glcbeta1,4GlcNAc-oxazoline as the donor substrate to glycosylate Asn-linked GlcNAc, while it was unable to recognize Manbeta1,4GlcNAc and Man(3)GlcNAc-oxazolines as the donor substrates. The chitinase-catalyzed transglycosylation was successfully extended to the remodeling of ribonuclease B to afford neoglycoproteins. Although the yield needs to be optimized, the chitinase-catalyzed transglycosylation provides a potentially useful tool for the synthesis of neoglycoproteins carrying novel N-linked oligosaccharides.
Figures



Similar articles
-
Synthesis of neoglycoproteins using oligosaccharide-transfer activity with endo-beta-N-acetylglucosaminidase.J Biol Chem. 1995 Feb 17;270(7):3094-9. doi: 10.1074/jbc.270.7.3094. J Biol Chem. 1995. PMID: 7852391
-
Transglycosylation reaction of a chitinase purified from Nocardia orientalis.Biochim Biophys Acta. 1987 Feb 20;923(2):302-9. doi: 10.1016/0304-4165(87)90017-1. Biochim Biophys Acta. 1987. PMID: 3814620
-
Chemoenzymatic synthesis of neoglycoproteins using transglycosylation with endo-beta-N-acetylglucosaminidase A.Biochem Biophys Res Commun. 2001 Apr 6;282(3):678-82. doi: 10.1006/bbrc.2001.4631. Biochem Biophys Res Commun. 2001. PMID: 11401514
-
Enzymatic properties of wild-type and active site mutants of chitinase A from Vibrio carchariae, as revealed by HPLC-MS.FEBS J. 2005 Jul;272(13):3376-86. doi: 10.1111/j.1742-4658.2005.04753.x. FEBS J. 2005. PMID: 15978043
-
Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation.Carbohydr Res. 2008 Jul 21;343(10-11):1509-22. doi: 10.1016/j.carres.2008.03.025. Epub 2008 Mar 27. Carbohydr Res. 2008. PMID: 18405887 Free PMC article. Review.
Cited by
-
Transglycosylation by chitinase D from Serratia proteamaculans improved through altered substrate interactions.J Biol Chem. 2012 Dec 28;287(53):44619-27. doi: 10.1074/jbc.M112.400879. Epub 2012 Oct 31. J Biol Chem. 2012. PMID: 23115231 Free PMC article.
-
Endo-beta-N-acetylglucosaminidase-catalyzed polymerization of beta-Glcp-(1-->4)-GlcpNAc oxazoline: a revisit to enzymatic transglycosylation.Carbohydr Res. 2009 Mar 31;344(5):592-8. doi: 10.1016/j.carres.2009.01.016. Epub 2009 Jan 19. Carbohydr Res. 2009. PMID: 19193364 Free PMC article.
References
-
- Bortone K, Monzingo AF, Ernst S, Robertus JD. J. Mol. Biol. 2002;320:293–302. - PubMed
-
- Papanikolau Y, Prag G, Tavlas G, Vorgias CE, Oppenheim AB, Petratos K. Biochemistry. 2001;40:11338–11343. - PubMed
-
- Terwisscha van Scheltinga AC, Armand S, Kalk KH, Isogai A, Henrissat B, Dijkstra BW. Biochemistry. 1995;34:15619–15623. - PubMed
-
- Tews I, Terwisscha van Scheltinga AC, Perrakis A, Wilson KS, Dijkstra BW. J. Am. Chem. Soc. 1997;119:7954–7959.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

