Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan
- PMID: 18784076
- PMCID: PMC2581576
- DOI: 10.1074/jbc.M806350200
Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan
Abstract
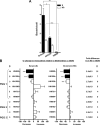
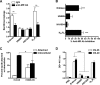
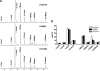
Phenotypically distinct clinical isolates of Mycobacterium tuberculosis are capable of altering the balance that exists between the pathogen and human host and ultimately the outcome of infection. This study has identified two M. tuberculosis strains (i.e. HN885 and HN1554) among a bank of clinical isolates with a striking defect in phagocytosis by primary human macrophages when compared with strain Erdman, a commonly used laboratory strain for studies of pathogenesis. Mass spectrometry in conjunction with NMR studies unequivocally confirmed that both HN885 and HN1554 contain truncated and more branched forms of mannose-capped lipoarabinomannan (ManLAM) with a marked reduction of their linear arabinan (corresponding mainly to the inner Araf-alpha(1-->5)-Araf unit) and mannan (with fewer 6-Manp residues and more substitutions in the linear Manp-alpha(1-->6)-Manp unit) domains. The truncation in the ManLAM molecules produced by strains HN885 and HN1554 led to a significant reduction in their surface availability. In addition, there was a marked reduction of higher order phosphatidyl-myo-inositol mannosides and the presence of dimycocerosates, triglycerides, and phenolic glycolipid in their cell envelope. Less exposed ManLAM and reduced higher order phosphatidyl-myo-inositol mannosides in strains HN885 and HN1554 resulted in their low association with the macrophage mannose receptor. Despite reduced phagocytosis, ingested bacilli replicated at a fast rate following serum opsonization. Our results provide evidence that the clinical spectrum of tuberculosis may be dictated not only by the host but also by the amounts and ratios of surface exposed mycobacterial adherence factors defined by strain genotype.
Figures

Similar articles
-
Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages.J Immunol. 1994 Apr 15;152(8):4070-9. J Immunol. 1994. PMID: 8144972
-
Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry.Glycobiology. 1993 Oct;3(5):497-506. doi: 10.1093/glycob/3.5.497. Glycobiology. 1993. PMID: 8286863
-
Lipoarabinomannan of Mycobacterium: mannose capping by a multifunctional terminal mannosyltransferase.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17973-7. doi: 10.1073/pnas.0807761105. Epub 2008 Nov 12. Proc Natl Acad Sci U S A. 2008. PMID: 19004785 Free PMC article.
-
Relationships between the structure and the roles of lipoarabinomannans and related glycoconjugates in tuberculosis pathogenesis.Front Biosci. 1998 Aug 6;3:e149-63. doi: 10.2741/a372. Front Biosci. 1998. PMID: 9696885 Review.
-
Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jun 1;76(4):fty026. doi: 10.1093/femspd/fty026. Pathog Dis. 2018. PMID: 29722821 Free PMC article. Review.
Cited by
-
Downregulation of protein kinase C-alpha enhances intracellular survival of Mycobacteria: role of PknG.BMC Microbiol. 2009 Dec 24;9:271. doi: 10.1186/1471-2180-9-271. BMC Microbiol. 2009. PMID: 20030858 Free PMC article.
-
Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses.J Biol Chem. 2011 Oct 14;286(41):35438-35446. doi: 10.1074/jbc.M111.232587. Epub 2011 Aug 22. J Biol Chem. 2011. PMID: 21859718 Free PMC article.
-
Mycobacterium Tuberculosis Infection and Inflammation: what is Beneficial for the Host and for the Bacterium?Front Microbiol. 2011 Jan 26;2:2. doi: 10.3389/fmicb.2011.00002. eCollection 2011. Front Microbiol. 2011. PMID: 21687401 Free PMC article.
-
Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells.PLoS One. 2012;7(8):e42515. doi: 10.1371/journal.pone.0042515. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880012 Free PMC article.
-
Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival.PLoS Pathog. 2010 Jul 8;6(7):e1000988. doi: 10.1371/journal.ppat.1000988. PLoS Pathog. 2010. PMID: 20628579 Free PMC article.
References
-
- World Health Organization (2007) Global Tuberculosis control surveillance, planning, and financing, World Health Organization Press, Geneva, Switzerland
-
- Torrelles, J. B., Azad, A. K., and Schlesinger, L. S. (2006) J. Immunol. 177 1805–1816 - PubMed
-
- Martinez-Pomares, L., Linehan, S. A., Taylor, P. R., and Gordon, S. (2001) Immunobiology 204 527–535 - PubMed
-
- Briken, V., Porcelli, S. A., Besra, G. S., and Kremer, L. (2004) Mol. Microbiol. 53 391–403 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

