Inactive and active states of the interferon-inducible resistance GTPase, Irga6, in vivo
- PMID: 18784077
- PMCID: PMC2581590
- DOI: 10.1074/jbc.M804846200
Inactive and active states of the interferon-inducible resistance GTPase, Irga6, in vivo
Abstract
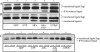
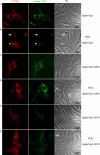
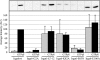
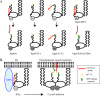
Irga6, a myristoylated, interferon-inducible member of the immunity-related GTPase family, contributes to disease resistance against Toxoplasma gondii in mice. Accumulation of Irga6 on the T. gondii parasitophorous vacuole membrane is associated with vesiculation and ultimately disruption of the vacuolar membrane in a process that requires an intact GTP-binding domain. The role of the GTP-binding domain of Irga6 in pathogen resistance is, however, unclear. We provide evidence that Irga6 in interferon-induced, uninfected cells is predominantly in a GDP-bound state that is maintained by other interferon-induced proteins. However, Irga6 that accumulates on the parasitophorous vacuole membrane after Toxoplasma infection is in the GTP-bound form. We demonstrate that a monoclonal antibody, 10D7, specifically detects GTP-bound Irga6, and we show that the formation of the 10D7 epitope follows from a GTP-dependent conformational transition of the N terminus of Irga6, anticipating an important role of the myristoyl group on Irga6 function in vivo.
Figures





Similar articles
-
The activation mechanism of Irga6, an interferon-inducible GTPase contributing to mouse resistance against Toxoplasma gondii.BMC Biol. 2011 Jan 28;9:7. doi: 10.1186/1741-7007-9-7. BMC Biol. 2011. PMID: 21276251 Free PMC article.
-
The immunity-related GTPase Irga6 dimerizes in a parallel head-to-head fashion.BMC Biol. 2016 Mar 2;14:14. doi: 10.1186/s12915-016-0236-7. BMC Biol. 2016. PMID: 26934976 Free PMC article.
-
RabGDIα is a negative regulator of interferon-γ-inducible GTPase-dependent cell-autonomous immunity to Toxoplasma gondii.Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):E4581-90. doi: 10.1073/pnas.1510031112. Epub 2015 Aug 3. Proc Natl Acad Sci U S A. 2015. PMID: 26240314 Free PMC article.
-
Toxoplasma gondii and the Immunity-Related GTPase (IRG) resistance system in mice: a review.Mem Inst Oswaldo Cruz. 2009 Mar;104(2):234-40. doi: 10.1590/s0074-02762009000200016. Mem Inst Oswaldo Cruz. 2009. PMID: 19430648 Review.
-
The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens.Mamm Genome. 2011 Feb;22(1-2):43-54. doi: 10.1007/s00335-010-9293-3. Epub 2010 Oct 30. Mamm Genome. 2011. PMID: 21052678 Free PMC article. Review.
Cited by
-
The mouse resistance protein Irgm1 (LRG-47): a regulator or an effector of pathogen defense?PLoS Pathog. 2010 Jul 15;6(7):e1001008. doi: 10.1371/journal.ppat.1001008. PLoS Pathog. 2010. PMID: 20664789 Free PMC article. No abstract available.
-
Human IRGM gene "to be or not to be".Semin Immunopathol. 2010 Dec;32(4):437-44. doi: 10.1007/s00281-010-0224-x. Epub 2010 Aug 25. Semin Immunopathol. 2010. PMID: 20737271 Review.
-
ROP39 is an Irgb10-specific parasite effector that modulates acute Toxoplasma gondii virulence.PLoS Pathog. 2023 Jan 5;19(1):e1011003. doi: 10.1371/journal.ppat.1011003. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36603017 Free PMC article.
-
Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):1126-31. doi: 10.1073/pnas.1313501111. Epub 2014 Jan 3. Proc Natl Acad Sci U S A. 2014. PMID: 24390541 Free PMC article.
-
Compensatory T cell responses in IRG-deficient mice prevent sustained Chlamydia trachomatis infections.PLoS Pathog. 2011 Jun;7(6):e1001346. doi: 10.1371/journal.ppat.1001346. Epub 2011 Jun 23. PLoS Pathog. 2011. PMID: 21731484 Free PMC article.
References
-
- Feng, C. G., Collazo-Custodio, C. M., Eckhaus, M., Hieny, S., Belkaid, Y., Elkins, K., Jankovic, D., Taylor, G. A., and Sher, A. (2004) J. Immunol. 172 1163-1168 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

