BK virus large T and VP-1 expression in infected human renal allografts
- PMID: 18784088
- PMCID: PMC2639064
- DOI: 10.1093/ndt/gfn470
BK virus large T and VP-1 expression in infected human renal allografts
Abstract
Objective: We investigated the expression of early and late phase BK virus (BKV) proteins and their interactions with host cell proteins in renal allografts, with ongoing polyomavirus associated nephropathy (PVAN), and correlated this with the nuclear and cell morphology.
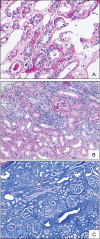
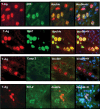
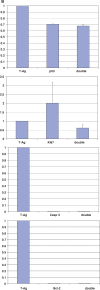
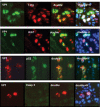
Methods: Frozen sections from three patients with renal allografts (two biopsies, one explant) with PVAN were analysed by indirect immunofluorescence using BKV specific anti-polyoma large T-antigen and anti-VP-1 antibodies, as well as anti-p53, anti-Ki67, anti-caspase-3, anti-bcl2 and anti-cytokeratin 22 antibodies. Nuclear morphology and size were estimated by DNA Hoechst staining.
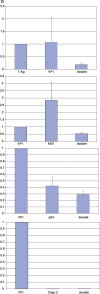
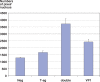
Results: In infected tubular cells the early and late phases of infection could be distinguished according to expression of large T-antigen or VP-1. The early phase revealed almost normal nuclear proportions, whereas in later phases nuclear size increased about 2 to 3 fold. Expression of large T-antigen was strongly associated with accumulation of p53 in the nucleus, accompanied by the activation of the cell cycle associated cell protein Ki67. In contrast, expression of BKV VP1 correlated only weakly with p53. Virus dependent cell lysis was due to necrosis, since neither caspase 3 nor nuclear nor cytoskeleton changes indicated apoptosis.
Conclusion: In our selected patients with PVAN a clear distinction between early and late phases was possible, according to the protein expression patterns of BKV markers. Striking nuclear enlargement is only present in the late phase of infection. In the inflammatory setting of PVAN, BKV dependent effects appear to be mediated by the inhibition of p53, resulting in the activation of the cell cycle. We assume that in PVAN similar BKV mechanisms are operative as in certain in vitro systems.
Figures

Similar articles
-
The status of BK polyomavirus replication in adult renal transplant recipients in northeastern Poland.Transplant Proc. 2011 Oct;43(8):2976-84. doi: 10.1016/j.transproceed.2011.07.009. Transplant Proc. 2011. PMID: 21996205
-
Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation.Transplantation. 2007 Aug 15;84(3):323-30. doi: 10.1097/01.tp.0000269706.59977.a5. Transplantation. 2007. PMID: 17700156
-
Polyomavirus polymerase chain reaction as a surrogate marker of polyomavirus-associated nephropathy.Transplantation. 2007 Aug 15;84(3):340-5. doi: 10.1097/01.tp.0000275205.41078.51. Transplantation. 2007. PMID: 17700158
-
[BK-virus and pathophysiology of associated diseases].Virologie (Montrouge). 2019 Feb 1;23(1):7-22. doi: 10.1684/vir.2019.0757. Virologie (Montrouge). 2019. PMID: 31131830 Review. French.
-
[BK viral infection after renal transplantation].Vnitr Lek. 2008 Sep;54(9):835-41. Vnitr Lek. 2008. PMID: 18924344 Review. Czech.
Cited by
-
Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in young kidney transplant patients.J Clin Microbiol. 2009 Aug;47(8):2577-85. doi: 10.1128/JCM.00030-09. Epub 2009 May 27. J Clin Microbiol. 2009. PMID: 19474265 Free PMC article.
-
BK Polyomavirus and the Transplanted Kidney: Immunopathology and Therapeutic Approaches.Transplantation. 2016 Nov;100(11):2276-2287. doi: 10.1097/TP.0000000000001333. Transplantation. 2016. PMID: 27391196 Free PMC article. Review.
-
Molecular networks involved in the immune control of BK polyomavirus.Clin Dev Immunol. 2012;2012:972102. doi: 10.1155/2012/972102. Epub 2012 Dec 5. Clin Dev Immunol. 2012. PMID: 23251224 Free PMC article.
-
Allogeneic CD4 T Cells Sustain Effective BK Polyomavirus-Specific CD8 T Cell Response in Kidney Transplant Recipients.Kidney Int Rep. 2024 May 7;9(8):2498-2513. doi: 10.1016/j.ekir.2024.04.070. eCollection 2024 Aug. Kidney Int Rep. 2024. PMID: 39156165 Free PMC article.
-
Modelling BK Polyomavirus dissemination and cytopathology using polarized human renal tubule epithelial cells.PLoS Pathog. 2023 Aug 28;19(8):e1011622. doi: 10.1371/journal.ppat.1011622. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37639485 Free PMC article.
References
-
- Gardner SD, Field AM, Coleman DV, et al. New human papovavirus (BK) isolated from urine after renal transplantation. Lancet. 1971;1:1253–1257. - PubMed
-
- Binet I, Nickeleit V, Hirsch HH, et al. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918–922. - PubMed
-
- Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999;10:1080–1089. - PubMed
-
- Purighalla R, Shapiro R, McCauley J, et al. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26:671–673. - PubMed
-
- Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

