Chemical substructures that enrich for biological activity
- PMID: 18784118
- PMCID: PMC2732283
- DOI: 10.1093/bioinformatics/btn479
Chemical substructures that enrich for biological activity
Abstract
Motivation: Certain chemical substructures are present in many drugs. This has led to the claim of 'privileged' substructures which are predisposed to bioactivity. Because bias in screening library construction could explain this phenomenon, the existence of privilege has been controversial.
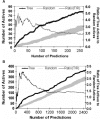
Results: Using diverse phenotypic assays, we defined bioactivity for multiple compound libraries. Many substructures were associated with bioactivity even after accounting for substructure prevalence in the library, thus validating the privileged substructure concept. Determinations of privilege were confirmed in independent assays and libraries. Our analysis also revealed 'underprivileged' substructures and 'conditional privilege'-rules relating combinations of substructure to bioactivity. Most previously reported substructures have been flat aromatic ring systems. Although we validated such substructures, we also identified three-dimensional privileged substructures. Most privileged substructures display a wide variety of substituents suggesting an entropic mechanism of privilege. Compounds containing privileged substructures had a doubled rate of bioactivity, suggesting practical consequences for pharmaceutical discovery.
Figures


Similar articles
-
Are target-family-privileged substructures truly privileged?J Med Chem. 2006 Mar 23;49(6):2000-9. doi: 10.1021/jm0502900. J Med Chem. 2006. PMID: 16539387
-
Chemical substructures in drug discovery.Drug Discov Today. 2003 Jul 1;8(13):594-602. doi: 10.1016/s1359-6446(03)02740-5. Drug Discov Today. 2003. PMID: 12850335 Review.
-
A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases.J Comb Chem. 1999 Jan;1(1):55-68. doi: 10.1021/cc9800071. J Comb Chem. 1999. PMID: 10746014
-
Synthesis of 3,5-Disubstituted Isoxazoles Containing Privileged Substructures with a Diverse Display of Polar Surface Area.ACS Comb Sci. 2017 Jun 12;19(6):407-413. doi: 10.1021/acscombsci.7b00032. Epub 2017 Mar 23. ACS Comb Sci. 2017. PMID: 28306232
-
Strategies for designing GPCR-focused libraries and screening sets.Curr Opin Drug Discov Devel. 2004 May;7(3):325-33. Curr Opin Drug Discov Devel. 2004. PMID: 15216936 Review.
Cited by
-
Gram matrix: an efficient representation of molecular conformation and learning objective for molecular pretraining.Brief Bioinform. 2024 May 23;25(4):bbae340. doi: 10.1093/bib/bbae340. Brief Bioinform. 2024. PMID: 38990515 Free PMC article.
-
Transition-Metal-Free Oxidative Cross-Coupling of Tetraarylborates to Biaryls Using Organic Oxidants.Angew Chem Int Ed Engl. 2020 Sep 1;59(36):15468-15473. doi: 10.1002/anie.202002595. Epub 2020 Apr 24. Angew Chem Int Ed Engl. 2020. PMID: 32159264 Free PMC article.
-
Fingerprint-Based Machine Learning Approach to Identify Potent and Selective 5-HT2BR Ligands.Molecules. 2018 May 10;23(5):1137. doi: 10.3390/molecules23051137. Molecules. 2018. PMID: 29748476 Free PMC article.
-
Antimicrobial Isoflavones and Derivatives from Erythrina (Fabaceae): Structure Activity Perspective (Sar & Qsar) on Experimental and Mined Values Against Staphylococcus Aureus.Antibiotics (Basel). 2020 Apr 30;9(5):223. doi: 10.3390/antibiotics9050223. Antibiotics (Basel). 2020. PMID: 32365905 Free PMC article.
-
Enhancing HCV NS3 Inhibitor Classification with Optimized Molecular Fingerprints Using Random Forest.Int J Mol Sci. 2025 Mar 17;26(6):2680. doi: 10.3390/ijms26062680. Int J Mol Sci. 2025. PMID: 40141322 Free PMC article.
References
-
- Andrews PR, Lloyd EJ. Molecular conformation and biological activity of central nervous system active drugs. Med. Res. Rev. 1982;2:355–393. - PubMed
-
- Ariens EJ, et al. The Receptors, a Comprehensive Treatise. New York: Plenum Press; 1979.
-
- Bemis GW, Murcko MA. The properties of known drugs. 1. Molecular frameworks. J. Med. Chem. 1996;39:2887–2893. - PubMed
-
- Bondensgaard K, et al. Recognition of privileged structures by G-protein coupled receptors. J. Med. Chem. 2004;47:888–899. - PubMed
-
- Boyce M, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

