Activation of the MAPK module from different spatial locations generates distinct system outputs
- PMID: 18784252
- PMCID: PMC2575182
- DOI: 10.1091/mbc.e08-04-0407
Activation of the MAPK module from different spatial locations generates distinct system outputs
Abstract
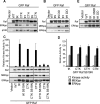
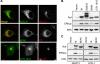
The Ras/Raf/MEK/ERK (MAPK) pathway directs multiple cell fate decisions within a single cell. How different system outputs are generated is unknown. Here we explore whether activating the MAPK module from different membrane environments can rewire system output. We identify two classes of nanoscale environment within the plasma membrane. The first, which corresponds to nanoclusters occupied by GTP-loaded H-, N- or K-Ras, supports Raf activation and amplifies low Raf kinase input to generate a digital ERKpp output. The second class, which corresponds to nanoclusters occupied by GDP-loaded Ras, cannot activate Raf and therefore does not activate the MAPK module, illustrating how lateral segregation on plasma membrane influences signal output. The MAPK module is activated at the Golgi, but in striking contrast to the plasma membrane, ERKpp output is analog. Different modes of Raf activation precisely correlate with these different ERKpp system outputs. Intriguingly, the Golgi contains two distinct membrane environments that generate ERKpp, but only one is competent to drive PC12 cell differentiation. The MAPK module is not activated from the ER. Taken together these data clearly demonstrate that the different nanoscale environments available to Ras generate distinct circuit configurations for the MAPK module, bestowing cells with a simple mechanism to generate multiple system outputs from a single cascade.
Figures



References
-
- Bivona T. G., Perez De Castro I., Ahearn I. M., Grana T. M., Chiu V. K., Lockyer P. J., Cullen P. J., Pellicer A., Cox A. D., Philips M. R. Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature. 2003;424:694–698. - PubMed
-
- Chiu V. K., Bivona T., Hach A., Sajous J. B., Silletti J., Wiener H., Johnson R. L., 2nd, Cox A. D., Philips M. R. Ras signalling on the endoplasmic reticulum and the Golgi. Nat. Cell Biol. 2002;4:343–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

