Yeast Golgi-localized, gamma-Ear-containing, ADP-ribosylation factor-binding proteins are but adaptor protein-1 is not required for cell-free transport of membrane proteins from the trans-Golgi network to the prevacuolar compartment
- PMID: 18784256
- PMCID: PMC2575161
- DOI: 10.1091/mbc.e07-05-0442
Yeast Golgi-localized, gamma-Ear-containing, ADP-ribosylation factor-binding proteins are but adaptor protein-1 is not required for cell-free transport of membrane proteins from the trans-Golgi network to the prevacuolar compartment
Abstract
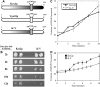
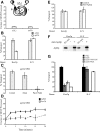
Golgi-localized, gamma-Ear-containing, ADP-ribosylation factor-binding proteins (GGAs) and adaptor protein-1 (AP-1) mediate clathrin-dependent trafficking of transmembrane proteins between the trans-Golgi network (TGN) and endosomes. In yeast, the vacuolar sorting receptor Vps10p follows a direct pathway from the TGN to the late endosome/prevacuolar compartment (PVC), whereas, the processing protease Kex2p partitions between the direct pathway and an indirect pathway through the early endosome. To examine the roles of the Ggas and AP-1 in TGN-PVC transport, we used a cell-free assay that measures delivery to the PVC of either Kex2p or a chimeric protein (K-V), in which the Vps10p cytosolic tail replaces the Kex2p tail. Either antibody inhibition or dominant-negative Gga2p completely blocked K-V transport but only partially blocked Kex2p transport. Deletion of APL2, encoding the beta subunit of AP-1, did not affect K-V transport but partially blocked Kex2p transport. Residual Kex2p transport seen with apl2Delta membranes was insensitive to dominant-negative Gga2p, suggesting that the apl2Delta mutation causes Kex2p to localize to a compartment that precludes Gga-dependent trafficking. These results suggest that yeast Ggas facilitate the specific and direct delivery of Vps10p and Kex2p from the TGN to the PVC and that AP-1 modulates Kex2p trafficking through a distinct pathway, presumably involving the early endosome.
Figures
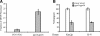

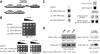

References
-
- Abazeed M. E., Blanchette J. M., Fuller R. S. Cell-free transport from the trans-Golgi network to late endosome requires factors involved in formation and consumption of clathrin-coated vesicles. J. Biol. Chem. 2005;280:4442–4450. - PubMed
-
- Blanchette J. M., Abazeed M. E., Fuller R. S. Cell-free reconstitution of transport from the trans-Golgi network to the late endosome/prevacuolar compartment. J. Biol. Chem. 2004;279:48767–48773. - PubMed
-
- Bonifacino J. S. The GGA proteins: adaptors on the move. Nat. Rev. Mol. Cell Biol. 2004;5:23–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

