Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition
- PMID: 18784296
- PMCID: PMC2729467
- DOI: 10.1523/JNEUROSCI.1789-08.2008
Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition
Abstract
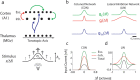
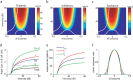
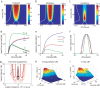
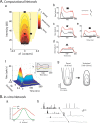
The frequency-intensity receptive fields (RF) of neurons in primary auditory cortex (AI) are heterogeneous. Some neurons have V-shaped RFs, whereas others have enclosed ovoid RFs. Moreover, there is a wide range of temporal response profiles ranging from phasic to tonic firing. The mechanisms underlying this diversity of receptive field properties are yet unknown. Here we study the characteristics of thalamocortical (TC) and intracortical connectivity that give rise to the individual cell responses. Using a mouse auditory TC slice preparation, we found that the amplitude of synaptic responses in AI varies non-monotonically with the intensity of the stimulation in the medial geniculate nucleus (MGv). We constructed a network model of MGv and AI that was simulated using either rate model cells or in vitro neurons through an iterative procedure that used the recorded neural responses to reconstruct network activity. We compared the receptive fields and firing profiles obtained with networks configured to have either cotuned excitatory and inhibitory inputs or relatively broad, lateral inhibitory inputs. Each of these networks yielded distinct response properties consistent with those documented in vivo with natural stimuli. The cotuned network produced V-shaped RFs, phasic-tonic firing profiles, and predominantly monotonic rate-level functions. The lateral inhibitory network produced enclosed RFs with narrow frequency tuning, a variety of firing profiles, and robust non-monotonic rate-level functions. We conclude that both types of circuits must be present to account for the wide variety of responses observed in vivo.
Figures


Similar articles
-
Auditory thalamocortical transmission is reliable and temporally precise.J Neurophysiol. 2005 Sep;94(3):2019-30. doi: 10.1152/jn.00860.2004. Epub 2005 May 31. J Neurophysiol. 2005. PMID: 15928054
-
Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body.Neuroscience. 2002;113(4):957-74. doi: 10.1016/s0306-4522(02)00240-3. Neuroscience. 2002. PMID: 12182900
-
Refinement of Spatial Receptive Fields in the Developing Mouse Lateral Geniculate Nucleus Is Coordinated with Excitatory and Inhibitory Remodeling.J Neurosci. 2018 May 9;38(19):4531-4542. doi: 10.1523/JNEUROSCI.2857-17.2018. Epub 2018 Apr 16. J Neurosci. 2018. PMID: 29661964 Free PMC article.
-
Spatial profile of excitatory and inhibitory synaptic connectivity in mouse primary auditory cortex.J Neurosci. 2012 Apr 18;32(16):5609-19. doi: 10.1523/JNEUROSCI.5158-11.2012. J Neurosci. 2012. PMID: 22514322 Free PMC article.
-
Synaptic mechanisms underlying auditory processing.Curr Opin Neurobiol. 2006 Aug;16(4):371-6. doi: 10.1016/j.conb.2006.06.015. Epub 2006 Jul 13. Curr Opin Neurobiol. 2006. PMID: 16842988 Review.
Cited by
-
Estradiol selectively enhances auditory function in avian forebrain neurons.J Neurosci. 2012 Dec 5;32(49):17597-611. doi: 10.1523/JNEUROSCI.3938-12.2012. J Neurosci. 2012. PMID: 23223283 Free PMC article.
-
Characterization of thalamocortical responses of regular-spiking and fast-spiking neurons of the mouse auditory cortex in vitro and in silico.J Neurophysiol. 2012 Mar;107(5):1476-88. doi: 10.1152/jn.00208.2011. Epub 2011 Nov 16. J Neurophysiol. 2012. PMID: 22090462 Free PMC article.
-
Preceding inhibition silences layer 6 neurons in auditory cortex.Neuron. 2010 Mar 11;65(5):706-17. doi: 10.1016/j.neuron.2010.02.021. Neuron. 2010. PMID: 20223205 Free PMC article.
-
Frequency transformation in the auditory lemniscal thalamocortical system.Front Neural Circuits. 2014 Jul 8;8:75. doi: 10.3389/fncir.2014.00075. eCollection 2014. Front Neural Circuits. 2014. PMID: 25071456 Free PMC article. Review.
-
Dynamics of the Auditory Continuity Illusion.Front Comput Neurosci. 2021 Jun 8;15:676637. doi: 10.3389/fncom.2021.676637. eCollection 2021. Front Comput Neurosci. 2021. PMID: 34168547 Free PMC article.
References
-
- Abeles M, Goldstein MH., Jr Responses of single units in the primary auditory cortex of the cat to tones and to tone pairs. Brain Res. 1972;42:337–352. - PubMed
-
- Aitkin LM, Webster WR. Medial geniculate body of the cat: organization and responses to tonal stimuli of neurons in ventral division. J Neurophysiol. 1972;35:365–380. - PubMed
-
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–1237. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
