A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal
- PMID: 18784298
- PMCID: PMC2615813
- DOI: 10.1523/JNEUROSCI.2783-08.2008
A novel Na+ channel splice form contributes to the regulation of an androgen-dependent social signal
Abstract
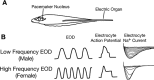
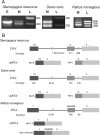
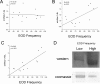
Na(+) channels are often spliced but little is known about the functional consequences of splicing. We have been studying the regulation of Na(+) current inactivation in an electric fish model in which systematic variation in the rate of inactivation of the electric organ Na(+) current shapes the electric organ discharge (EOD), a sexually dimorphic, androgen-sensitive communication signal. Here, we examine the relationship between an Na(+) channel (Na(v)1.4b), which has two splice forms, and the waveform of the EOD. One splice form (Na(v)1.4bL) possesses a novel first exon that encodes a 51 aa N-terminal extension. This is the first report of an Na(+) channel with alternative splicing in the N terminal. This N terminal is present in zebrafish suggesting its general importance in regulating Na(+) currents in teleosts. The extended N terminal significantly speeds fast inactivation, shifts steady-state inactivation, and dramatically enhances recovery from inactivation, essentially fulfilling the functions of a beta subunit. Both splice forms are equally expressed in muscle in electric fish and zebrafish but Na(v)1.4bL is the dominant form in the electric organ implying electric organ-specific transcriptional regulation. Transcript abundance of Na(v)1.4bL in the electric organ is positively correlated with EOD frequency and lowered by androgens. Thus, shaping of the EOD waveform involves the androgenic regulation of a rapidly inactivating splice form of an Na(+) channel. Our results emphasize the role of splicing in the regulation of a vertebrate Na(+) channel and its contribution to a known behavior.
Figures

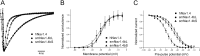





Similar articles
-
Individual variation and hormonal modulation of a sodium channel beta subunit in the electric organ correlate with variation in a social signal.Dev Neurobiol. 2007 Sep 1;67(10):1289-304. doi: 10.1002/dneu.20404. Dev Neurobiol. 2007. PMID: 17638382
-
Alternative splicing in the voltage-gated sodium channel DmNav regulates activation, inactivation, and persistent current.J Neurophysiol. 2009 Sep;102(3):1994-2006. doi: 10.1152/jn.00613.2009. Epub 2009 Jul 22. J Neurophysiol. 2009. PMID: 19625535 Free PMC article.
-
Individual variation in and androgen-modulation of the sodium current in electric organ.J Neurosci. 1995 May;15(5 Pt 2):4023-32. doi: 10.1523/JNEUROSCI.15-05-04023.1995. J Neurosci. 1995. PMID: 7751963 Free PMC article.
-
Androgen modulates the kinetics of the delayed rectifying K+ current in the electric organ of a weakly electric fish.Dev Neurobiol. 2007 Oct;67(12):1589-97. doi: 10.1002/dneu.20530. Dev Neurobiol. 2007. PMID: 17562532
-
Molecular evolution of communication signals in electric fish.J Exp Biol. 2008 Jun;211(Pt 11):1814-8. doi: 10.1242/jeb.015982. J Exp Biol. 2008. PMID: 18490397 Review.
Cited by
-
Behavioral ecology, endocrinology and signal reliability of electric communication.J Exp Biol. 2013 Jul 1;216(Pt 13):2403-11. doi: 10.1242/jeb.082255. J Exp Biol. 2013. PMID: 23761465 Free PMC article. Review.
-
A neuroendocrine basis for the hierarchical control of frog courtship vocalizations.Front Neuroendocrinol. 2011 Aug;32(3):353-66. doi: 10.1016/j.yfrne.2010.12.006. Epub 2010 Dec 28. Front Neuroendocrinol. 2011. PMID: 21192966 Free PMC article. Review.
-
Frank Beach Award Winner: Steroids as neuromodulators of brain circuits and behavior.Horm Behav. 2014 Aug;66(3):552-60. doi: 10.1016/j.yhbeh.2014.07.014. Epub 2014 Aug 7. Horm Behav. 2014. PMID: 25110187 Free PMC article. Review.
-
Ionic mechanisms of microsecond-scale spike timing in single cells.J Neurosci. 2014 May 7;34(19):6668-78. doi: 10.1523/JNEUROSCI.0615-14.2014. J Neurosci. 2014. PMID: 24806692 Free PMC article.
-
Evidence for Non-neutral Evolution in a Sodium Channel Gene in African Weakly Electric Fish (Campylomormyrus, Mormyridae).J Mol Evol. 2016 Aug;83(1-2):61-77. doi: 10.1007/s00239-016-9754-8. Epub 2016 Aug 1. J Mol Evol. 2016. PMID: 27481396
References
-
- Akopian AN, Okuse K, Souslova V, England S, Ogata N, Wood JN. Trans-splicing of a voltage-gated sodium channel is regulated by nerve growth factor. FEBS Lett. 1999;445:177–182. - PubMed
-
- Blumenstein Y, Kanevsky N, Sahar G, Barzilai R, Ivanina T, Dascal N. A novel long N-terminal isoform of human L-type Ca2+ channel is up-regulated by protein kinase C. J Biol Chem. 2002;277:3419–3423. - PubMed
-
- Bohm RA, Wang B, Brenner R, Atkinson NS. Transcriptional control of Ca(2+)-activated K(+) channel expression: identification of a second, evolutionarily conserved, neuronal promoter. J Exp Biol. 2000;203:693–704. - PubMed
-
- Chew LJ, Gallo V. Regulation of ion channel expression in neural cells by hormones and growth factors. Mol Neurobiol. 1998;18:175–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
