Noggin expands neural stem cells in the adult hippocampus
- PMID: 18784300
- PMCID: PMC3651371
- DOI: 10.1523/JNEUROSCI.3314-07.2008
Noggin expands neural stem cells in the adult hippocampus
Abstract
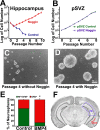
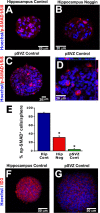
New neurons are added to the adult hippocampus throughout life and contribute to cognitive functions, including learning and memory. It remains unclear whether ongoing neurogenesis arises from self-renewing neural stem cells (NSCs) or from multipotential progenitor cells that cannot self-renew in the hippocampus. This is primarily based on observations that neural precursors derived from the subventricular zone (SVZ) can be passaged long term, whereas hippocampal subgranular zone (SGZ) precursors are rapidly depleted by passaging. We demonstrate here that high levels of bone morphogenetic protein (BMP) signaling occur in hippocampal but not SVZ precursors in vitro, and blocking BMP signaling with Noggin is sufficient to foster hippocampal cell self-renewal, proliferation, and multipotentiality using single-cell clonal analysis. Moreover, NSC maintenance requires continual Noggin exposure, which implicates BMPs as crucial regulators of NSC aging. In vivo, Noggin is expressed in the adult dentate gyrus and limits BMP signaling in proliferative cells of the SGZ. Transgenic Noggin overexpression in the SGZ increases multiple precursor cell populations but proportionally increases the glial fibrillary acidic protein-positive cell population at the expense of other precursors, suggesting that Noggin acts on NSCs in vivo. To confirm this, we used a dual thymidine analog paradigm to repeatedly label slowly dividing cells over a long duration. We find that small populations of label-retaining cells exist in the SGZ and that Noggin overexpression increases their numbers. Thus, we propose that the adult hippocampus contains a population of NSCs, which can be expanded both in vitro and in vivo by blocking BMP signaling.
Figures





Similar articles
-
A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo.Eur J Neurosci. 2007 Dec;26(11):3024-35. doi: 10.1111/j.1460-9568.2007.05940.x. Eur J Neurosci. 2007. PMID: 18028109
-
Consequences of noggin expression by neural stem, glial, and neuronal precursor cells engrafted into the injured spinal cord.Exp Neurol. 2005 Oct;195(2):293-304. doi: 10.1016/j.expneurol.2005.04.021. Exp Neurol. 2005. PMID: 16087174
-
Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory.J Neurosci. 2011 Jul 6;31(27):9933-44. doi: 10.1523/JNEUROSCI.1062-11.2011. J Neurosci. 2011. PMID: 21734285 Free PMC article.
-
A developmental perspective on adult hippocampal neurogenesis.Int J Dev Neurosci. 2013 Nov;31(7):640-5. doi: 10.1016/j.ijdevneu.2013.04.001. Epub 2013 Apr 12. Int J Dev Neurosci. 2013. PMID: 23588197 Review.
-
Survey of transcriptome analyses of hippocampal neurogenesis with focus on adult dentate gyrus stem cells.Front Cell Dev Biol. 2025 May 30;13:1605116. doi: 10.3389/fcell.2025.1605116. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519263 Free PMC article. Review.
Cited by
-
β1-integrin restricts astrocytic differentiation of adult hippocampal neural stem cells.Glia. 2016 Jul;64(7):1235-51. doi: 10.1002/glia.22996. Epub 2016 May 5. Glia. 2016. PMID: 27145730 Free PMC article.
-
The generation of definitive neural stem cells from PiggyBac transposon-induced pluripotent stem cells can be enhanced by induction of the NOTCH signaling pathway.Stem Cells Dev. 2013 Feb 1;22(3):383-96. doi: 10.1089/scd.2012.0218. Epub 2012 Sep 17. Stem Cells Dev. 2013. PMID: 22889305 Free PMC article.
-
Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain.Front Cell Neurosci. 2013 Jan 18;6:70. doi: 10.3389/fncel.2012.00070. eCollection 2012. Front Cell Neurosci. 2013. PMID: 23346046 Free PMC article.
-
Soluble amyloid precursor protein-α rescues age-linked decline in neural progenitor cell proliferation.Neurobiol Aging. 2013 Oct;34(10):2431-40. doi: 10.1016/j.neurobiolaging.2013.04.016. Epub 2013 May 14. Neurobiol Aging. 2013. PMID: 23683827 Free PMC article.
-
Stem cell-based models and therapies for neurodegenerative diseases.Crit Rev Biomed Eng. 2009;37(4-5):321-53. doi: 10.1615/critrevbiomedeng.v37.i4-5.20. Crit Rev Biomed Eng. 2009. PMID: 20528730 Free PMC article. Review.
References
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
