Hierarchical organization of human cortical networks in health and schizophrenia
- PMID: 18784304
- PMCID: PMC2878961
- DOI: 10.1523/JNEUROSCI.1929-08.2008
Hierarchical organization of human cortical networks in health and schizophrenia
Abstract
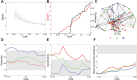
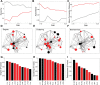
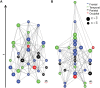
The complex organization of connectivity in the human brain is incompletely understood. Recently, topological measures based on graph theory have provided a new approach to quantify large-scale cortical networks. These methods have been applied to anatomical connectivity data on nonhuman species, and cortical networks have been shown to have small-world topology, associated with high local and global efficiency of information transfer. Anatomical networks derived from cortical thickness measurements have shown the same organizational properties of the healthy human brain, consistent with similar results reported in functional networks derived from resting state functional magnetic resonance imaging (MRI) and magnetoencephalographic data. Here we show, using anatomical networks derived from analysis of inter-regional covariation of gray matter volume in MRI data on 259 healthy volunteers, that classical divisions of cortex (multimodal, unimodal, and transmodal) have some distinct topological attributes. Although all cortical divisions shared nonrandom properties of small-worldness and efficient wiring (short mean Euclidean distance between connected regions), the multimodal network had a hierarchical organization, dominated by frontal hubs with low clustering, whereas the transmodal network was assortative. Moreover, in a sample of 203 people with schizophrenia, multimodal network organization was abnormal, as indicated by reduced hierarchy, the loss of frontal and the emergence of nonfrontal hubs, and increased connection distance. We propose that the topological differences between divisions of normal cortex may represent the outcome of different growth processes for multimodal and transmodal networks and that neurodevelopmental abnormalities in schizophrenia specifically impact multimodal cortical organization.
Figures

References
-
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
