Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells
- PMID: 18784379
- PMCID: PMC2584985
- DOI: 10.1152/ajpcell.00075.2008
Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells
Abstract
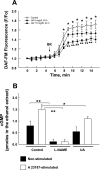
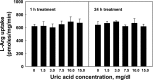
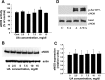
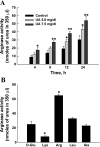
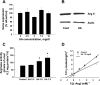
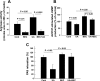
Elevated levels of serum uric acid (UA) are commonly associated with primary pulmonary hypertension but have generally not been thought to have any causal role. Recent experimental studies, however, have suggested that UA may affect various vasoactive mediators. We therefore tested the hypothesis that UA might alter nitric oxide (NO) levels in pulmonary arterial endothelial cells (PAEC). In isolated porcine pulmonary artery segments (PAS), UA (7.5 mg/dl) inhibits acetylcholine-induced vasodilation. The incubation of PAEC with UA caused a dose-dependent decrease in NO and cGMP production stimulated by bradykinin or Ca(2+)-ionophore A23187. We explored cellular mechanisms by which UA might cause reduced NO production focusing on the effects of UA on the l-arginine-endothelial NO synthase (eNOS) and l-arginine-arginase pathways. Incubation of PAEC with different concentrations of UA (2.5-15 mg/dl) for 24 h did not affect l-[(3)H]arginine uptake or activity/expression of eNOS. However, PAEC incubated with UA (7.5 mg/dl; 24 h) released more urea in culture media than control PAEC, suggesting that arginase activation might be involved in the UA effect. Kinetic analysis of arginase activity in PAEC lysates and rat liver and kidney homogenates demonstrated that UA activated arginase by increasing its affinity for l-arginine. An inhibitor of arginase (S)-(2-boronoethyl)-l-cysteine prevented UA-induced reduction of A23187-stimulated cGMP production by PAEC and abolished UA-induced inhibition of acetylcholine-stimulated vasodilation in PAS. We conclude that UA-induced arginase activation is a potential mechanism for reduction of NO production in PAEC.
Figures

References
-
- Aubert JD Biochemical markers in the management of pulmonary hypertension. Swiss Med Wkly 135: 43–49, 2005. - PubMed
-
- Bendayan D, Shitrit D, Ygla M, Huerta M, Fink G, Kramer M. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Resp Med 97: 130–133, 2003. - PubMed
-
- Berkowitz D, White R, Li D, Minhas K, Cernetich A, Kim S, Burke S, Shoukas A, Nyhan D, Champion H, Hare J. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 108: 2000–2006, 2003. - PubMed
-
- Block ER, Patel J, Sheridan N. Endotoxin protects against hyperoxic decrease in membrane fluidity in endothelial cells but not in fibroblasts. Lab Invest 54: 146–153, 1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

