Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy
- PMID: 18784459
- PMCID: PMC2828871
- DOI: 10.1097/QAD.0b013e32830efd79
Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy
Abstract
Introduction: Data on initial antiretroviral regimen longevity predates the arrival of newer nucleoside reverse transcriptase inhibitor backbones and once-daily regimens. Modern regimens are thought to possess greater tolerability and convenience. We hypothesized this would translate into greater durability.
Methods: Retrospective study of antiretroviral-naive patients starting treatment at the University of Alabama at Birmingham 1917 HIV/AIDS Clinic 1 January 2000-31 July 2007. Two periods of antiretroviral initiation were identified, prior and after August 2004 (arrival of once-daily fixed-dose regimens). Kaplan-Meier survival analyses of regimen durability by time period and regimen characteristics were performed. Staged Cox proportional hazards models evaluated the roles of dosing complexity and composition in explaining differences in regimen durability between study periods.
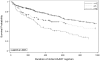
Results: Overall 542 patients started antiretroviral drugs (n = 309, January 2000-July 2004; n = 233, August 2004-July 2007). Median durability was 263 days longer in after August 2004 regimens. Regimens started before August 2004 had increased hazards for discontinuation relative to after August 2004 regimens [hazard ratio (HR) = 1.44; 95% confidence interval (CI) = 1.03-2.02]. Time period of initiation lost statistical significance when the model included dosing frequency (HR = 1.92 for at least twice daily vs. daily; 95% CI = 1.29-2.88). As regimen composition variables were added, time period and dosing frequency lost significance. Increased hazards of discontinuation were observed with didanosine or stavudine relative to abacavir or tenofovir use (HR = 1.92; 95% CI = 1.29-2.88) and all third drugs compared with non-nucleoside reverse transcriptase inhibitor-based regimens (triple-nucleoside reverse transcriptase inhibitor HR = 1.76; 95% CI = 1.14-2.73; unboosted-protease inhibitor HR = 1.58; 95% CI = 1.02-2.46; boosted-protease inhibitor HR = 1.57; 95% CI = 1.02-2.41). Affective mental health disorders increased the hazard of discontinuation in all models.
Conclusion: Durability of contemporary once-daily fixed-dose antiretroviral regimens has significantly eclipsed the duration of earlier antiretroviral drug options. Our results indicate this is due to both more convenient dosing and improved tolerability of modern antiretroviral regimens.
Figures




References
-
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. - PubMed
-
- Jacobson JM, Turner BJ, Abrutyn E. Trials that matter: CD4+ T-lymphocyte count-guided interruption of antiretroviral therapy in HIV-infected patients. Ann Intern Med. 2007;146:682–683. - PubMed
-
- Ruiz L, Paredes R, Gomez G, Romeu J, Domingo P, Perez-Alvarez N, et al. Antiretroviral therapy interruption guided by CD4 cell counts and plasma HIV-1 RNA levels in chronically HIV-1-infected patients. Aids. 2007;21:169–178. - PubMed
-
- Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. Jama. 2006;296:827–843. - PubMed
-
- d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. Aids. 2000;14:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

