Ethanol enhances glutamate transmission by retrograde dopamine signaling in a postsynaptic neuron/synaptic bouton preparation from the ventral tegmental area
- PMID: 18784647
- PMCID: PMC2761034
- DOI: 10.1038/npp.2008.143
Ethanol enhances glutamate transmission by retrograde dopamine signaling in a postsynaptic neuron/synaptic bouton preparation from the ventral tegmental area
Abstract
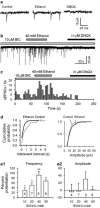
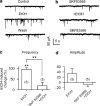
It is well documented that somatodendritically released dopamine is important in the excitability and synaptic transmission of midbrain dopaminergic neurons. Recently we showed that in midbrain slices, acute ethanol exposure facilitates glutamatergic transmission onto dopaminergic neurons in the ventral tegmental area (VTA). The VTA is a brain region critical to the rewarding effects of abused drugs, including ethanol. We hypothesized that ethanol facilitation might result from an increase in somatodendritically released dopamine, which acts retrogradely on dopamine D(1) receptors on glutamate-releasing axons and consequently leads to an increase in glutamate release onto dopaminergic neurons. To further test this hypothesis and to examine whether ethanol facilitation can occur at the single-cell level, VTA neurons were freshly isolated from rat brains using an enzyme-free procedure. These isolated neurons retain functional synaptic terminals, including those that release glutamate. Spontaneous excitatory postsynaptic currents (sEPSCs) mediated by glutamate alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors were recorded from these freshly isolated putative dopaminergic neurons. We found that acute application of clinically relevant concentrations of ethanol (10-80 mM) significantly facilitated the frequency of sEPSCs but not their mean amplitude. Ethanol facilitation was mimicked by the D(1) agonist SKF 38393 and by the dopamine uptake blocker GBR 12935 but was blocked by the D(1) antagonist SKF 83566, and by depleting dopamine stores with reserpine, as well as by chelating postsynaptic calcium with BAPTA. Furthermore, the sodium channel blocker tetrodotoxin eliminated the facilitation of sEPSCs induced by ethanol but not by SKF 38393. These results constitute the first evidence from single isolated cells of ethanol facilitation of glutamate transmission to dopaminergic neurons in the VTA. In addition, we show that ethanol facilitation has a postsynaptic origin and a presynaptic locus. Furthermore, ethanol stimulation of a single dopaminergic neuron is capable of eliciting the release of somatodendritic dopamine, which is sufficient to influence glutamatergic transmission at individual synapses.
Figures





References
-
- Abarca J, Gysling K, Roth RH, Bustos G. Changes in extracellular levels of glutamate and aspartate in rat substantia nigra induced by dopamine receptor ligands: in vivo micro-dialysis studies. Neurochem Res. 1995;20:159–169. - PubMed
-
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. - PubMed
-
- Aghajanian GK, Bunney BS. Pharmacological characterization of dopamine ‘autoreceptors’ by microiontophoretic single-cell recording studies. Adv Biochem Psychopharmacol. 1977;16:433–438. - PubMed
-
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

