Exacerbation of apoptosis of cortical neurons following traumatic brain injury in par-4 transgenic mice
- PMID: 18784822
- PMCID: PMC2480534
Exacerbation of apoptosis of cortical neurons following traumatic brain injury in par-4 transgenic mice
Abstract
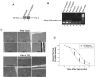
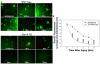
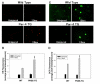
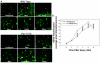
Traumatic brain injury (TBI) is a significant clinical problem, yet few effective strategies for treating it have emerged. People that sustain and survive a TBI are left with significant cognitive, behavioral, and communicative disabilities. Apoptotic neuronal death occurs following TBI. Prostate apoptosis response-4 (Par-4) is a death domain-containing protein initially characterized as a critical regulator of apoptosis in prostate cancer cells. We have recently generated and characterized Par-4 transgenic mice in which the expression of the par-4 transgene was limited to cells of neuronal lineage. We now provide evidence that, in cortical neurons from these mice, Par-4 drastically increases apoptotic neuronal death in both in vitro and in vivo models of TBI. In vitro experiments were performed in 7-day-old primary cultures of cortical neurons using a previously published, scratch-induced mechanical trauma model. Neurons that overexpress Par-4 showed not only a significant decrease in overall neuron survival after TBI compared to wild-type cells, but also exhibited a sharper decrease in mitochondrial transmembrane potential, a higher degree of free radical accumulation, and earlier activation of caspase-3 than wild-type cells did. In vivo experiments were performed utilizing a weight drop TBI model. A significantly increased volume of cortical injury and exacerbated activation of caspase-3 were observed in Par-4 transgenic mice when compared to those in wild-type mice. These data suggests that aberrant Par-4 expression exacerbates neuronal cell death following TBI by altering mitochondrial function, enhancing oxidative damage, and execution of apoptosis via caspase activation.
Keywords: Traumatic brain injury; apoptosis; cell culture; cerebral cortex; prostate apoptosis response-4.
Figures


References
-
- DeFord SM, Wilson MS, Rice AC, Clausen T, Rice LK, Barabnova A, Bullock R, Hamm RJ. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J Neurotrauma. 2002;19:427–438. - PubMed
-
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007;85:805–815. - PubMed
-
- Vink R, Nimmo AJ, Cernak I. An overview of new and novel pharmacotherapies for use in traumatic brain injury. Clin Exp Pharmacol Physiol. 2001;28:919–921. - PubMed
-
- Hatton J. Pharmacological treatment of traumatic brain injury: a review of agents in development. CNS Drugs. 2001;15:553–581. - PubMed
-
- Wong J, Hoe NW, Zhiwei F, Ng I. Apoptosis and traumatic brain injury. Neurocrit Care. 2005;3:177–182. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
