Epstein-Barr virus and gastric carcinoma--viral carcinogenesis through epigenetic mechanisms
- PMID: 18784828
- PMCID: PMC2480567
Epstein-Barr virus and gastric carcinoma--viral carcinogenesis through epigenetic mechanisms
Abstract
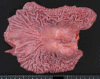
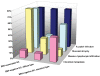
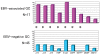
Epstein-Barr virus (EBV)-associated gastric carcinoma (GC) is the monoclonal growth of EBV-infected epithelial cells, and the entity was recognized only recently. EBV-associated GC is distributed worldwide and more than 90,000 patients are estimated to develop GC annually in association with EBV (10% of total GC). EBV-associated GC occurs in two forms in terms of the histological features, i.e., lymphoepithelioma-like GC and ordinary type of GC. Both share characteristic clinicopathological features, such as the preferential occurrence as multiple cancer and remnant stomach cancer. While the expression of EBV-latent genes is restricted to several in the infected cells (Latency I), EBV-associated GC shows gastric cell phenotype, resistance to apoptosis, and the production of immunomodulator molecules. Recently, global and non-random CpG island methylation of the promoter region of many cancer-related genes has been demonstrated with their decreased expression, such as p16 INK4A, p73 and E-cadherin. This abnormality is accompanied by methylation of the EBV genome itself, suggesting a process of virus-driven hypermethylation in the development of neoplastic cells. Further studies are necessary to determine the precise sequence of EBV infection, methylation, transformation and selection of the predominant clone within the stomach mucosa. Future studies are also desirable for the target and strategy of therapy, such as initiating viral replication or reversing the DNA methylation of cellular genes.
Keywords: DNA methylation; Epstein-Barr virus; chronic inflammation; gastric cancer; histology; viral oncogenesis.
Figures
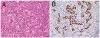




References
-
- Epstein MA. Reflections on Epstein-Barr virus: some recently resolved old uncertainties. J Infect. 2001;43:111–115. - PubMed
-
- Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377–380. - PubMed
-
- Min KW, Holmquist S, Peiper SC, O'Leary TJ. Poorly differentiated adenocarcinoma with lymphoid stroma (lymphoepithelioma-like carcinomas) of the stomach. Report of three cases with Epstein-Barr virus genome demonstrated by the polymerase chain reaction. Am J Clin Pathol. 1991;96:219–227. - PubMed
-
- Tokunaga M, Uemura Y, Tokudome T, Ishidate T, Masuda H, Okazaki E, Kaneko K, Naoe S, Ito M, Okamura A, et al. Epstein-Barr virus related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol Jpn. 1993;43:574–581. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
