Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats
- PMID: 18785000
- PMCID: PMC11506079
- DOI: 10.1007/s10571-008-9308-8
Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats
Abstract
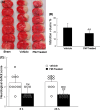
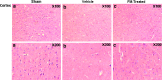
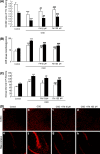
We previously reported that inhibition of Rho-kinase (ROCK) by hydroxyl fasudil improves cognitive deficit and neuronal damage in rats with chronic cerebral ischemia (Huang et al., Cell Mol Neurobiol 28:757-768, 2008). In this study, fasudil mesylate (FM) was investigated for its neuroprotective potential in rats with ischemia following middle cerebral artery occlusion (MCAO) and reperfusion. The effect of fasudil mesylate was also studied in rat brain cortical and hippocampal slices treated with oxygen-glucose deprivation (OGD) injury. Gross anatomy showed that cerebral infarct size, measured with 2,3,5-triphenyltetrazolium chloride (TTC) staining, was significantly smaller in the FM-treated than in the non-FM-treated ischemic rats. In the brain regions vulnerable to ischemia of ischemic rats, fasudil mesylate was also found to significantly restore the enzyme protein expression level of endothelial nitric oxide synthase (eNOS), which was decreased in ischemia. However, it remarkably reduced the protein synthesis of inducible nitric oxide synthase (iNOS) that was induced by ischemia and reperfusion. In rat brain slices treated with OGD injury, fasudil mesylate increased the neuronal cell viability by 40% for cortex and by 61% for hippocampus, respectively. Finally, in the presence of OGD and fasudil mesylate, superoxide dismutase (SOD) activity was increased by 50% for cortex and by 58% for hippocampus, compared to OGD only group. In conclusion, our in vivo study showed that fasudil mesylate not only decreased neurological deficit but also reduced cerebral infarct size, possibly and at least partially by augmenting eNOS protein expression and inhibiting iNOS protein expression after ischemia-reperfusion.
Figures



References
-
- Cohen MM, Pettegrew JW, Kopp SJ, Minshew N, Glonek T (1984) P-31 nuclear magnetic resonance analysis of brain: normoxic and anoxic brain slices. Neurochem Res 9:785–801. doi:10.1007/BF00965666 - PubMed
-
- Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J (1999) Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 30:2631–2636 - PubMed
-
- Dawson VL, Brahmbhatt HP, Mong JA, Dawson TM (1994) Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology 33:1425–1430. doi:10.1016/0028-3908(94)90045-0 - PubMed
-
- De AJ, Cardenas A, Moro MA, Leza JC, Lorenzo P, Lizasoain I (1999) Use of brain slices in the study of pathogenic role of inducible nitric oxide synthase in cerebral ischemia-reperfusion. Gen Pharmacol 32:577–581. doi:10.1016/S0306-3623(98)00280-8 - PubMed
-
- Dobashi K, Araki S, Kubo K, Kawagoe R, Yamamoto Y, Shirahata A (2008) Hydroxymethylglutaryl-CoA reductase inhibitor inhibits induction of nitric oxide synthase in 3T3-L1 preadipocytes. Life Sci 82:85–90. doi:10.1016/j.lfs.2007.10.013 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

