Changes in apical dendritic structure correlate with sustained ERK1/2 phosphorylation in medial prefrontal cortex of a rat model of dopamine D1 receptor agonist sensitization
- PMID: 18785628
- PMCID: PMC2587500
- DOI: 10.1002/cne.21835
Changes in apical dendritic structure correlate with sustained ERK1/2 phosphorylation in medial prefrontal cortex of a rat model of dopamine D1 receptor agonist sensitization
Abstract
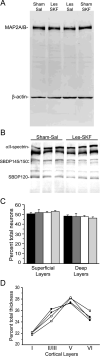
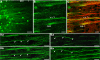
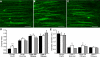
Rats lesioned with 6-hydroxydopamine (6-OHDA) as neonates exhibit behavioral and neurochemical abnormalities in adulthood that mimic Lesch-Nyhan disease, schizophrenia, and other developmental disorders of frontostriatal circuit dysfunction. In these animals a latent sensitivity to D1 agonists is maximally exposed by repeated administration of dopamine agonists in the postpubertal period (D1 priming). In neonate-lesioned, adult rats primed with SKF-38393, we found selective, persistent alterations in the morphology of pyramidal neuron apical dendrites in the prelimbic area of the medial prefrontal cortex (mPFC). In these animals, dendrite bundling patterns and the typically straight trajectories of primary dendritic shafts were disrupted, whereas the diameter of higher-order oblique branches was increased. Although not present in neonate-lesioned rats treated with saline, these morphological changes persisted at least 21 days after repeated dosing with SKF-38393, and were not accompanied by markers of neurodegenerative change. A sustained increase in phospho-ERK immunoreactivity in wavy dendritic shafts over the same period suggested a relationship between prolonged ERK phosphorylation and dendritic remodeling in D1-primed rats. In support of this hypothesis, pretreatment with the MEK1/2-ERK1/2 pathway inhibitors PD98059 or SL327, prior to each priming dose of SKF-38393, prevented the morphological changes associated with D1 priming. Together, these findings demonstrate that repeated stimulation of D1 receptors in adulthood interacts with the developmental loss of dopamine to profoundly and persistently modify neuronal signaling and dendrite morphology in the mature prefrontal cortex. Furthermore, sustained elevation of ERK activity in mPFC pyramidal neurons may play a role in guiding these morphological changes in vivo.
(c) 2008 Wiley-Liss, Inc.
Figures



Similar articles
-
Sustained extracellular signal-regulated kinase 1/2 phosphorylation in neonate 6-hydroxydopamine-lesioned rats after repeated D1-dopamine receptor agonist administration: implications for NMDA receptor involvement.J Neurosci. 2004 Jun 30;24(26):5863-76. doi: 10.1523/JNEUROSCI.0528-04.2004. J Neurosci. 2004. PMID: 15229233 Free PMC article.
-
Synergistic interactions of dopamine D1 and glutamate NMDA receptors in rat hippocampus and prefrontal cortex: involvement of ERK1/2 signaling.Neuroscience. 2009 Nov 10;163(4):1135-45. doi: 10.1016/j.neuroscience.2009.07.056. Epub 2009 Jul 30. Neuroscience. 2009. PMID: 19647050
-
Prior D1 dopamine receptor stimulation is required to prime D2-mediated striatal Fos expression in 6-hydroxydopamine-lesioned rats.Neuroscience. 1999;94(2):505-14. doi: 10.1016/s0306-4522(99)00338-3. Neuroscience. 1999. PMID: 10579212
-
Repeated treatments with the D1 dopamine receptor agonist SKF-38393 modulate cell viability via sustained ERK-Bad-Bax activation in dopaminergic neuronal cells.Behav Brain Res. 2019 Jul 23;367:166-175. doi: 10.1016/j.bbr.2019.03.035. Epub 2019 Mar 28. Behav Brain Res. 2019. PMID: 30930179
-
Activation of extracellular signal-regulated protein kinases is associated with a sensitized locomotor response to D(2) dopamine receptor stimulation in unilateral 6-hydroxydopamine-lesioned rats.J Neurosci. 2000 Mar 1;20(5):1849-57. doi: 10.1523/JNEUROSCI.20-05-01849.2000. J Neurosci. 2000. PMID: 10684886 Free PMC article.
Cited by
-
Sucrose Consumption Alters Serotonin/Glutamate Co-localisation Within the Prefrontal Cortex and Hippocampus of Mice.Front Mol Neurosci. 2021 Jun 28;14:678267. doi: 10.3389/fnmol.2021.678267. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34262435 Free PMC article.
-
Convergence of Lemniscal and Local Excitatory Inputs on Large GABAergic Tectothalamic Neurons.J Comp Neurol. 2015 Oct 15;523(15):2277-96. doi: 10.1002/cne.23789. Epub 2015 May 12. J Comp Neurol. 2015. PMID: 25879870 Free PMC article.
-
Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism.Neuroscience. 2009 Mar 17;159(2):483-91. doi: 10.1016/j.neuroscience.2008.12.050. Epub 2009 Jan 3. Neuroscience. 2009. PMID: 19167462 Free PMC article.
-
Inversed Effects of Nav1.2 Deficiency at Medial Prefrontal Cortex and Ventral Tegmental Area for Prepulse Inhibition in Acoustic Startle Response.Mol Neurobiol. 2024 Feb;61(2):622-634. doi: 10.1007/s12035-023-03610-6. Epub 2023 Aug 31. Mol Neurobiol. 2024. PMID: 37650965
-
Activation of Extracellular Signal-Regulated Kinases (ERK 1/2) in the Locus Coeruleus Contributes to Pain-Related Anxiety in Arthritic Male Rats.Int J Neuropsychopharmacol. 2017 Jun 1;20(6):463. doi: 10.1093/ijnp/pyx005. Int J Neuropsychopharmacol. 2017. PMID: 28158734 Free PMC article.
References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Alpar A, Palm K, Schierwagen A, Arendt T, Gartner U. Expression of constitutively active p21H-rasval12 in postmitotic pyramidal neurons results in increased dendritic size and complexity. J Comp Neurol. 2003;467:119–133. - PubMed
-
- Arendt T. Neurodegeneration and plasticity. Int J Dev Neurosci. 2004;22:507–514. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
-
- Binder LI, Frankfurter A, Rebhun LI. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann NY Acad Sci. 1986;466:145–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

