Probing the pH-dependent prepore to pore transition of Bacillus anthracis protective antigen with differential oxidative protein footprinting
- PMID: 18785752
- PMCID: PMC2645544
- DOI: 10.1021/bi800533t
Probing the pH-dependent prepore to pore transition of Bacillus anthracis protective antigen with differential oxidative protein footprinting
Abstract
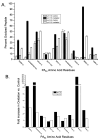
The protective antigen (PA) component of the anthrax toxin (ATx) plays an essential role in the pathogenesis of the bioterrorism bacterium Bacillus anthracis. After oligomerization on the cell surface and docking of lethal factor and/or edema factor, PA is internalized and undergoes a conformational change when exposed to the low pH of the endosome to form a membrane-penetrating pore. While the structure of the PA prepore has been determined, precise structural information regarding the pore state remains lacking. Oxidative protein footprinting (OPF) can provide dynamic structural information about a protein complex through analysis of amino acid oxidation both before and after a conformational change. In this study, PA at pH 7.5 and 5.5 was exposed to hydroxyl radicals generated by ionizing radiation. Mass spectrometry was then used to both identify and quantitate the extent of oxidation of differentially modified residues. Several residues were found to be more readily oxidized at pH 7.5, most of which clustered toward the bottom plane of the prepore heptamer. Two amino acids had greater oxidation rates at pH 5.5, both found on the outer periphery of the prepore. When the OPF results were mapped to a current computational model of the pore, the accessibilities of some residues were consistent with their modeled positions in the pore (i.e., Y688 and V619/I620), while data for other residues (W346 and M350) appeared to conflict with the model. The results from this study illustrate the utility of OPF in generating empirical structural information for yet undetermined structures and offering opportunities for refinement for models thereof.
Figures



References
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. - PubMed
-
- Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

