A covalent linker allows for membrane targeting of an oxylipin biosynthetic complex
- PMID: 18785758
- PMCID: PMC2665874
- DOI: 10.1021/bi800751p
A covalent linker allows for membrane targeting of an oxylipin biosynthetic complex
Abstract
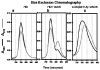
A naturally occurring bifunctional protein from Plexaura homomalla links sequential catalytic activities in an oxylipin biosynthetic pathway. The C-terminal lipoxygenase (LOX) portion of the molecule catalyzes the transformation of arachidonic acid (AA) to the corresponding 8 R-hydroperoxide, and the N-terminal allene oxide synthase (AOS) domain promotes the conversion of the hydroperoxide intermediate to the product allene oxide (AO). Small-angle X-ray scattering data indicate that in the absence of a covalent linkage the two catalytic domains that transform AA to AO associate to form a complex that recapitulates the structure of the bifunctional protein. The SAXS data also support a model for LOX and AOS domain orientation in the fusion protein inferred from a low-resolution crystal structure. However, results of membrane binding experiments indicate that covalent linkage of the domains is required for Ca (2+)-dependent membrane targeting of the sequential activities, despite the noncovalent domain association. Furthermore, membrane targeting is accompanied by a conformational change as monitored by specific proteolysis of the linker that joins the AOS and LOX domains. Our data are consistent with a model in which Ca (2+)-dependent membrane binding relieves the noncovalent interactions between the AOS and LOX domains and suggests that the C2-like domain of LOX mediates both protein-protein and protein-membrane interactions.
Figures




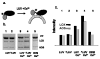
Similar articles
-
ALLENE OXIDE SYNTHASE and HYDROPEROXIDE LYASE, Two Non-Canonical Cytochrome P450s in Arabidopsis thaliana and Their Different Roles in Plant Defense.Int J Mol Sci. 2019 Jun 23;20(12):3064. doi: 10.3390/ijms20123064. Int J Mol Sci. 2019. PMID: 31234561 Free PMC article. Review.
-
Identification of a functional allene oxide synthase-lipoxygenase fusion protein in the soft coral Gersemia fruticosa suggests the generality of this pathway in octocorals.Biochim Biophys Acta. 2008 Feb;1780(2):315-21. doi: 10.1016/j.bbagen.2007.10.010. Epub 2007 Oct 22. Biochim Biophys Acta. 2008. PMID: 17996204
-
Role of radical formation at tyrosine 193 in the allene oxide synthase domain of a lipoxygenase-AOS fusion protein from coral.Biochemistry. 2003 Jun 10;42(22):6871-80. doi: 10.1021/bi027427y. Biochemistry. 2003. PMID: 12779342 Free PMC article.
-
Purification and catalytic activities of the two domains of the allene oxide synthase-lipoxygenase fusion protein of the coral Plexaura homomalla.J Biol Chem. 1999 Nov 19;274(47):33764-70. doi: 10.1074/jbc.274.47.33764. J Biol Chem. 1999. PMID: 10559269
-
Allene oxide synthases and allene oxides.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:423-31. doi: 10.1016/s0090-6980(02)00046-1. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432934 Review.
Cited by
-
ALLENE OXIDE SYNTHASE and HYDROPEROXIDE LYASE, Two Non-Canonical Cytochrome P450s in Arabidopsis thaliana and Their Different Roles in Plant Defense.Int J Mol Sci. 2019 Jun 23;20(12):3064. doi: 10.3390/ijms20123064. Int J Mol Sci. 2019. PMID: 31234561 Free PMC article. Review.
-
Expression of an 8R-Lipoxygenase From the Coral Plexaura homomalla.Methods Enzymol. 2018;605:33-49. doi: 10.1016/bs.mie.2018.02.010. Epub 2018 Mar 28. Methods Enzymol. 2018. PMID: 29909831 Free PMC article.
-
Structural and functional insights into the reaction specificity of catalase-related hydroperoxide lyase: A shift from lyase activity to allene oxide synthase by site-directed mutagenesis.PLoS One. 2017 Sep 27;12(9):e0185291. doi: 10.1371/journal.pone.0185291. eCollection 2017. PLoS One. 2017. PMID: 28953966 Free PMC article.
-
Substrate channeling in oxylipin biosynthesis through a protein complex in the plastid envelope of Arabidopsis thaliana.J Exp Bot. 2019 Mar 11;70(5):1483-1495. doi: 10.1093/jxb/erz015. J Exp Bot. 2019. PMID: 30690555 Free PMC article.
-
Catalase-Related Allene Oxide Synthase, on a Biosynthetic Route to Fatty Acid Cyclopentenones: Expression and Assay of the Enzyme and Preparation of the 8R-HPETE Substrate.Methods Enzymol. 2018;605:51-68. doi: 10.1016/bs.mie.2018.02.019. Epub 2018 May 2. Methods Enzymol. 2018. PMID: 29909837 Free PMC article.
References
-
- Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–40. - PubMed
-
- Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487:323–326. - PubMed
-
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. - PubMed
-
- Kuhn H, Thiele BJ. The diversity of the lipoxygenase family. Many sequence data but little information on biological significance. FEBS Lett. 1999;449:7–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

