Acute perturbations in Golgi organization impact de novo sphingomyelin synthesis
- PMID: 18785922
- PMCID: PMC2862547
- DOI: 10.1111/j.1600-0854.2008.00810.x
Acute perturbations in Golgi organization impact de novo sphingomyelin synthesis
Abstract
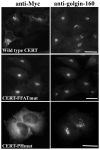
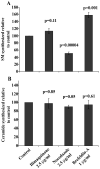
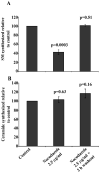
The mammalian Golgi apparatus is composed of multiple stacks of cisternal membranes organized laterally into a ribbon-like structure, with close apposition of trans Golgi regions with specialized endoplasmic reticulum (ER) membranes. These contacts may be the site of ceramide transfer from its site of synthesis (ER) to sphingomyelin (SM) synthase through ceramide transfer protein (CERT). CERT extracts ceramide from the ER and transfers it to Golgi membranes but the role of overall Golgi structure in this process is unknown. We show here that localization of CERT in puncta around the Golgi complex requires both ER- and Golgi-binding domains of CERT. To examine how Golgi structure contributes to SM synthesis, we treated cells with Golgi-perturbing drugs and measured newly synthesized SM. Interestingly, disruption of Golgi morphology with nocodazole, but not ilimaquinone inhibited SM synthesis. Decreased localization of CERT with a Golgi marker correlated with decreased SM synthesis. We propose that some Golgi structural perturbations interfere with efficient ceramide trafficking through CERT, and thus SM synthesis. The organization of the mammalian Golgi ribbon together with CERT may promote specific ER-Golgi interactions for efficient delivery of ceramide for SM synthesis.
Figures


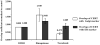
Similar articles
-
Inactivation of ceramide transfer protein during pro-apoptotic stress by Golgi disassembly and caspase cleavage.Biochem J. 2012 Mar 1;442(2):391-401. doi: 10.1042/BJ20111461. Biochem J. 2012. PMID: 22129459 Free PMC article.
-
Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development.PLoS Pathog. 2011 Sep;7(9):e1002198. doi: 10.1371/journal.ppat.1002198. Epub 2011 Sep 1. PLoS Pathog. 2011. PMID: 21909260 Free PMC article.
-
Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites.FEBS Lett. 2019 Sep;593(17):2366-2377. doi: 10.1002/1873-3468.13511. Epub 2019 Jul 8. FEBS Lett. 2019. PMID: 31254361 Review.
-
CERT and intracellular trafficking of ceramide.Biochim Biophys Acta. 2007 Jun;1771(6):644-53. doi: 10.1016/j.bbalip.2007.01.009. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17314061 Review.
-
CERT-mediated trafficking of ceramide.Biochim Biophys Acta. 2009 Jul;1791(7):684-91. doi: 10.1016/j.bbalip.2009.01.006. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19416656 Review.
Cited by
-
The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites.PLoS Pathog. 2011 Jun;7(6):e1002092. doi: 10.1371/journal.ppat.1002092. Epub 2011 Jun 23. PLoS Pathog. 2011. PMID: 21731489 Free PMC article.
-
The ceramide-enriched trans-Golgi compartments reorganize together with other parts of the Golgi apparatus in response to ATP-depletion.Histochem Cell Biol. 2011 Feb;135(2):159-71. doi: 10.1007/s00418-010-0773-z. Epub 2011 Jan 12. Histochem Cell Biol. 2011. PMID: 21225431
-
PKD controls mitotic Golgi complex fragmentation through a Raf-MEK1 pathway.Mol Biol Cell. 2013 Feb;24(3):222-33. doi: 10.1091/mbc.E12-03-0198. Epub 2012 Dec 14. Mol Biol Cell. 2013. PMID: 23242995 Free PMC article.
-
Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent Golgi fragmentation.PLoS Pathog. 2009 Oct;5(10):e1000615. doi: 10.1371/journal.ppat.1000615. Epub 2009 Oct 9. PLoS Pathog. 2009. PMID: 19816566 Free PMC article.
-
The Infectious Bronchitis Coronavirus Envelope Protein Alters Golgi pH To Protect the Spike Protein and Promote the Release of Infectious Virus.J Virol. 2019 May 15;93(11):e00015-19. doi: 10.1128/JVI.00015-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30867314 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

