Characterization of inhibitory mechanism and antifungal activity between group-1 and group-2 phytocystatins from taro (Colocasia esculenta)
- PMID: 18785929
- PMCID: PMC7164091
- DOI: 10.1111/j.1742-4658.2008.06631.x
Characterization of inhibitory mechanism and antifungal activity between group-1 and group-2 phytocystatins from taro (Colocasia esculenta)
Abstract
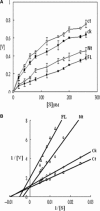
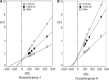
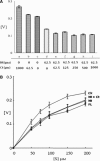
Tarocystatin from Colocasia esculenta, a group-2 phytocystatin, is a defense protein against phytopathogenic nematodes and fungi. It is composed of a highly conserved N-terminal region, which is homological to group-1 cystatin, and a repetitive peptide at the C-terminus. The purified recombinant proteins of tarocystatin, such as full-length (FL), N-terminus (Nt) and C-terminus (Ct) peptides, were produced and their inhibitory activities against papain as well as their antifungal effects were investigated. Kinetic analysis revealed that FL peptide exhibited mixed type inhibition (K(ia) = 0.098 microM and K(ib) = 0.252 microM) and Nt peptide showed competitive inhibition (K(i) = 0.057 microM), whereas Ct peptide possessed weak papain activation properties. A shift in the inhibitory pattern from competitive inhibition of Nt peptide alone to mixed type inhibition of FL peptide implied that the Ct peptide has an regulatory effect on the function of FL peptide. Based on the inhibitory kinetics of FL (group-2) and Nt (group-1) peptides on papain activity, an inhibitory mechanism of group-2 phytocystatins and a regulatory mechanism of extended Ct peptide have each been proposed. By contrast, the antifungal activity of Nt peptide appeared to be greater than that of FL peptide, and the Ct peptide showed no effect on antifungal activity, indicating that the antifungal effect is not related to proteinase inhibitory activity. The results are valid for most phytocystatins with respect to the inhibitory mechanism against cysteine proteinase.
Figures




Similar articles
-
Crystal structure of tarocystatin-papain complex: implications for the inhibition property of group-2 phytocystatins.Planta. 2011 Aug;234(2):243-54. doi: 10.1007/s00425-011-1398-8. Epub 2011 Mar 18. Planta. 2011. PMID: 21416241 Free PMC article.
-
A new Piper nigrum cysteine proteinase inhibitor, PnCPI, with antifungal activity: molecular cloning, recombinant expression, functional analyses and molecular modeling.Planta. 2020 Jul 13;252(2):16. doi: 10.1007/s00425-020-03425-y. Planta. 2020. PMID: 32661769
-
Molecular cloning, recombinant gene expression, and antifungal activity of cystatin from taro (Colocasia esculenta cv. Kaosiung no. 1).Planta. 2005 Jun;221(4):493-501. doi: 10.1007/s00425-004-1462-8. Epub 2005 Jan 13. Planta. 2005. PMID: 15647900
-
Phytocystatins: Defense Proteins against Phytophagous Insects and Acari.Int J Mol Sci. 2016 Oct 20;17(10):1747. doi: 10.3390/ijms17101747. Int J Mol Sci. 2016. PMID: 27775606 Free PMC article. Review.
-
Phytocystatins and their potential to control plant diseases caused by fungi.Protein Pept Lett. 2015;22(2):104-11. doi: 10.2174/0929866521666140418101711. Protein Pept Lett. 2015. PMID: 24746092 Review.
Cited by
-
Differential response of silencing HvIcy2 barley plants against Magnaporthe oryzae infection and light deprivation.BMC Plant Biol. 2018 Dec 6;18(1):337. doi: 10.1186/s12870-018-1560-6. BMC Plant Biol. 2018. PMID: 30522452 Free PMC article.
-
Crystal structure of tarocystatin-papain complex: implications for the inhibition property of group-2 phytocystatins.Planta. 2011 Aug;234(2):243-54. doi: 10.1007/s00425-011-1398-8. Epub 2011 Mar 18. Planta. 2011. PMID: 21416241 Free PMC article.
-
Genome-Wide Identification and Expression Analysis of the Protease Inhibitor Gene Families in Tomato.Genes (Basel). 2019 Dec 18;11(1):1. doi: 10.3390/genes11010001. Genes (Basel). 2019. PMID: 31861342 Free PMC article.
-
Rice bifunctional phytocystatin is a dual modulator of legumain and papain-like proteases.Plant Mol Biol. 2016 Sep;92(1-2):193-207. doi: 10.1007/s11103-016-0504-5. Epub 2016 Jun 20. Plant Mol Biol. 2016. PMID: 27325119
-
Theobroma cacao cystatins impair Moniliophthora perniciosa mycelial growth and are involved in postponing cell death symptoms.Planta. 2010 Nov;232(6):1485-97. doi: 10.1007/s00425-010-1272-0. Epub 2010 Sep 22. Planta. 2010. PMID: 20859638
References
-
- Margis R, Reis EM & Villeret V (1998) Structural and phylogenetic relationships among plant and animal cystatins. Arch Biochem Biophys 359, 24–30. - PubMed
-
- Machleidt W, Thiele M, Laber B, Assfalg‐Machleid I, Esterl A, Wiegand G, Kos J, Turk V & Bode W (1989) Mechanism of inhibition of papain by chicken egg white cystatin. FEBS Lett 243, 234–238. - PubMed
-
- Arai S, Watanabe H, Kondo H, Emori Y & Abe K (1991) Papain‐inhibitory activity of oryzacystatin, a rice seed cysteine proteinase inhibitor, depends on the central Gln‐Val‐Val‐Ala‐Gly region conserved among cystatin superfamily members. J Biochem (Tokyo) 109, 294–298. - PubMed
-
- Abe K, Emori Y, Kondo H, Suzuki K & Arai S (1987) Molecular cloning of a cysteine proteinase inhibitor of rice (oryzacystatin). Homology with animal cystatins and transient expression in the ripening process of rice seeds. J Biol Chem 262, 16793–16797. - PubMed
-
- Lim CO, Lee SI, Chung WS, Park SH, Hwang I & Cho MJ (1996) Characterization of a cDNA encoding a cysteine proteinase inhibitor from chinese cabbage (Brassica campestris L. ssp. pekinensis) flower buds. Plant Mol Biol 30, 373–379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

