Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment
- PMID: 18785974
- PMCID: PMC2612717
- DOI: 10.1111/j.1365-2249.2008.03753.x
Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment
Abstract
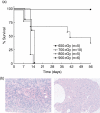
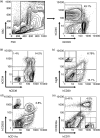
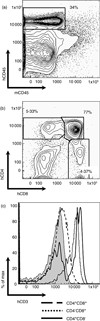
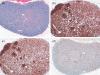
Immunodeficient hosts engrafted with human lymphohaematopoietic cells hold great promise as a preclinical bridge for understanding human haematopoiesis and immunity. We now describe a new immunodeficient radioresistant non-obese diabetic mice (NOD) stock based on targeted mutations in the recombination activating gene-1 (Rag1(null)) and interleukin (IL)-2 receptor common gamma chain (IL2rgamma(null)), and compare its ability to support lymphohaematopoietic cell engraftment with that achieved in radiosensitive NOD.CB17-Prkdc(scid) (NOD-Prkdc(scid)) IL2rgamma(null) mice. We observed that immunodeficient NOD-Rag1(null) IL2rgamma(null) mice tolerated much higher levels of irradiation conditioning than did NOD-Prkdc(scid) IL2rgamma(null) mice. High levels of human cord blood stem cell engraftment were observed in both stocks of irradiation-conditioned adult mice, leading to multi-lineage haematopoietic cell populations and a complete repertoire of human immune cells, including human T cells. Human peripheral blood mononuclear cells also engrafted at high levels in unconditioned adult mice of each stock. These data document that Rag1(null) and scid stocks of immunodeficient NOD mice harbouring the IL2rgamma(null) mutation support similar levels of human lymphohaematopoietic cell engraftment. NOD-Rag1(null) IL2rgamma(null) mice will be an important new model for human lymphohaematopoietic cell engraftment studies that require radioresistant hosts.
Figures




References
-
- Shultz LD, Pearson T, King M, et al. Humanized NOD/ltsz-scid il2 receptor common gamma chain knockout mice in diabetes research. Ann NY Acad Sci. 2007;1103:77–89. - PubMed
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev. 2007;7:118–30. - PubMed
-
- Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176:2053–8. - PubMed
-
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

