The circadian clock in the retina controls rod-cone coupling
- PMID: 18786362
- PMCID: PMC5581203
- DOI: 10.1016/j.neuron.2008.07.017
The circadian clock in the retina controls rod-cone coupling
Abstract
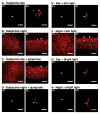
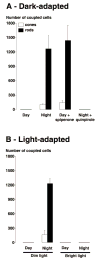
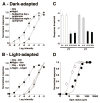
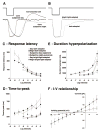
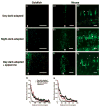
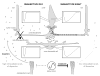
Although rod and cone photoreceptor cells in the vertebrate retina are anatomically connected or coupled by gap junctions, a type of electrical synapse, rod-cone electrical coupling is thought to be weak. Using tracer labeling and electrical recording in the goldfish retina and tracer labeling in the mouse retina, we show that the retinal circadian clock, and not the retinal response to the visual environment, controls the extent and strength of rod-cone coupling by activating dopamine D(2)-like receptors in the day, so that rod-cone coupling is weak during the day but remarkably robust at night. The results demonstrate that circadian control of rod-cone electrical coupling serves as a synaptic switch that allows cones to receive very dim light signals from rods at night, but not in the day. The increase in the strength and extent of rod-cone coupling at night may facilitate the detection of large dim objects.
Figures

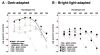
References
-
- Barlow RB. Circadian and efferent modulation of visual sensitivity. Prog Brain Res. 2001;131:487–503. - PubMed
-
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. - PubMed
-
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. - PubMed
-
- Burns ME, Arshavsky VY. Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron. 2005;48:387–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

