Temporal organization of the cell cycle
- PMID: 18786381
- PMCID: PMC2856080
- DOI: 10.1016/j.cub.2008.07.001
Temporal organization of the cell cycle
Abstract
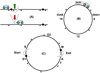
The coordination of growth, DNA replication and division in proliferating cells can be adequately explained by a 'clock + checkpoint' model. The clock is an underlying cyclical sequence of states; the checkpoints ensure that the cycle proceeds without mistakes. From the molecular complexities of the control system in modern eukaryotes, we isolate a simple network of positive and negative feedbacks that embodies a 'clock + checkpoints'. The model accounts for the fundamental physiological properties of mitotic cell divisions, evokes a new view of the meiotic program, and suggests how the control system may have evolved in the first place.
Figures






References
-
- Mitchison JM. The Biology of the Cell Cycle. Cambridge UK: Cambridge Univ. Press; 1971.
-
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. - PubMed
-
- Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. - PubMed
-
- Kelly TJ, Martin GS, Forsburg SL, Stephen RJ, Russo A, Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993;74:371–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

