Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain
- PMID: 18786414
- PMCID: PMC2613692
- DOI: 10.1016/j.stem.2008.07.004
Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain
Abstract
Neural stem cells (NSCs, B1 cells) are retained in the walls of the adult lateral ventricles but, unlike embryonic NSCs, are displaced from the ventricular zone (VZ) into the subventricular zone (SVZ) by ependymal cells. Apical and basal compartments, which in embryonic NSCs play essential roles in self-renewal and differentiation, are not evident in adult NSCs. Here we show that SVZ B1 cells in adult mice extend a minute apical ending to directly contact the ventricle and a long basal process ending on blood vessels. A closer look at the ventricular surface reveals a striking pinwheel organization specific to regions of adult neurogenesis. The pinwheel's core contains the apical endings of B1 cells and in its periphery two types of ependymal cells: multiciliated (E1) and a type (E2) characterized by only two cilia and extraordinarily complex basal bodies. These results reveal that adult NSCs retain fundamental epithelial properties, including apical and basal compartmentalization, significantly reshaping our understanding of this adult neurogenic niche.
Figures
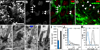
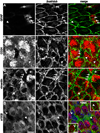
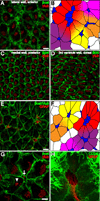
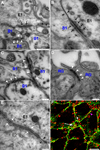
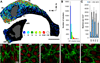
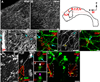
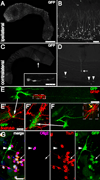

Comment in
-
The niche revealed.Cell Stem Cell. 2008 Sep 11;3(3):234-6. doi: 10.1016/j.stem.2008.08.011. Cell Stem Cell. 2008. PMID: 18786409 No abstract available.
References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. - PubMed
-
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation "hot spots" in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

