Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions
- PMID: 18786416
- PMCID: PMC2747473
- DOI: 10.1016/j.stem.2008.07.026
Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions
Abstract
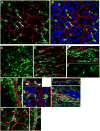
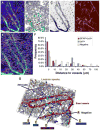
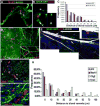
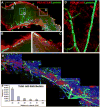
There is an emerging understanding of the importance of the vascular system within stem cell niches. Here, we examine whether neural stem cells (NSCs) in the adult subventricular zone (SVZ) lie close to blood vessels, using three-dimensional whole mounts, confocal microscopy, and automated computer-based image quantification. We found that the SVZ contains a rich plexus of blood vessels that snake along and within neuroblast chains. Cells expressing stem cell markers, including GFAP, and proliferation markers are closely apposed to the laminin-containing extracellular matrix (ECM) surrounding vascular endothelial cells. Apical GFAP+ cells are admixed within the ependymal layer and some span between the ventricle and blood vessels, occupying a specialized microenvironment. Adult SVZ progenitor cells express the laminin receptor alpha6beta1 integrin, and blocking this inhibits their adhesion to endothelial cells, altering their position and proliferation in vivo, indicating that it plays a functional role in binding SVZ stem cells within the vascular niche.
Figures


Comment in
-
The niche revealed.Cell Stem Cell. 2008 Sep 11;3(3):234-6. doi: 10.1016/j.stem.2008.08.011. Cell Stem Cell. 2008. PMID: 18786409 No abstract available.
References
-
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. - PubMed
-
- Breier G, Albrecht U, Sterrer S, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

