Robust axonal growth and a blunted macrophage response are associated with impaired functional recovery after spinal cord injury in the MRL/MpJ mouse
- PMID: 18786615
- PMCID: PMC2579759
- DOI: 10.1016/j.neuroscience.2008.08.013
Robust axonal growth and a blunted macrophage response are associated with impaired functional recovery after spinal cord injury in the MRL/MpJ mouse
Abstract
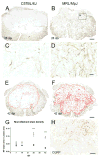
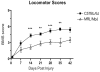
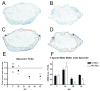
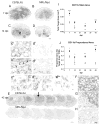
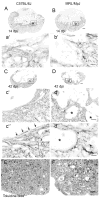
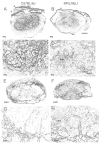
Spinal cord injury (SCI) in mammals leads to a robust inflammatory response followed by the formation of a glial and connective tissue scar that comprises a barrier to axonal regeneration. The inbred MRL/MpJ mouse strain exhibits reduced inflammation after peripheral injury and shows true regeneration without tissue scar formation following an ear punch wound. We hypothesized that following SCI, the unique genetic wound healing traits of this strain would result in reduced glial and connective tissue scar formation, increased axonal growth, and improved functional recovery. Adult MRL/MpJ and C57BL/6J mice were subjected to a mid-thoracic spinal contusion and the distribution of axon profiles and selected cellular and extracellular matrix components was compared at 1, 2, 4 and 6 weeks post-injury. Recovery of hind-limb locomotor function was assessed over the same time period. The MRL/MpJ mice exhibited robust axon growth within the lesion, beginning at 4 weeks post-injury. This growth was accompanied by reduced macrophage staining at 1, 2, 4 and 6 weeks post-injury, decreased chondroitin sulfate proteoglycan staining at 1-2 weeks and increased laminin staining throughout the lesion at 2-6 weeks post-injury. Paradoxically, the extent of locomotor recovery was impaired in the MRL/MpJ mice. Close examination of the chronic lesion site revealed evidence of ongoing degeneration both within and surrounding the lesion site. Thus, the regenerative genetic wound healing traits of the MRL/MpJ mice contribute to the evolution of a lesion environment that supports enhanced axon growth after SCI. However, this response occurs at the expense of meaningful functional recovery.
Figures


Similar articles
-
Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection.PLoS One. 2012;7(2):e30904. doi: 10.1371/journal.pone.0030904. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348029 Free PMC article.
-
Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response.J Comp Neurol. 2004 Jul 5;474(4):469-86. doi: 10.1002/cne.20149. J Comp Neurol. 2004. PMID: 15174067
-
Altered CNS response to injury in the MRL/MpJ mouse.Neuroscience. 2004;127(4):821-32. doi: 10.1016/j.neuroscience.2004.05.057. Neuroscience. 2004. PMID: 15312895
-
Moving beyond the glial scar for spinal cord repair.Nat Commun. 2019 Aug 28;10(1):3879. doi: 10.1038/s41467-019-11707-7. Nat Commun. 2019. PMID: 31462640 Free PMC article. Review.
-
Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury.Exp Neurol. 2008 Feb;209(2):378-88. doi: 10.1016/j.expneurol.2007.06.009. Epub 2007 Jun 30. Exp Neurol. 2008. PMID: 17662717 Free PMC article. Review.
Cited by
-
Complement C6 deficiency exacerbates pathophysiology after spinal cord injury.Sci Rep. 2020 Nov 11;10(1):19500. doi: 10.1038/s41598-020-76441-3. Sci Rep. 2020. PMID: 33177623 Free PMC article.
-
Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection.PLoS One. 2012;7(2):e30904. doi: 10.1371/journal.pone.0030904. Epub 2012 Feb 13. PLoS One. 2012. PMID: 22348029 Free PMC article.
-
Macrophages migrate in an activation-dependent manner to chemokines involved in neuroinflammation.J Neuroinflammation. 2014 Feb 1;11:23. doi: 10.1186/1742-2094-11-23. J Neuroinflammation. 2014. PMID: 24485070 Free PMC article.
-
Apelin alleviated neuroinflammation and promoted endogenous neural stem cell proliferation and differentiation after spinal cord injury in rats.J Neuroinflammation. 2022 Jun 20;19(1):160. doi: 10.1186/s12974-022-02518-7. J Neuroinflammation. 2022. PMID: 35725619 Free PMC article.
-
The glial scar in spinal cord injury and repair.Neurosci Bull. 2013 Aug;29(4):421-35. doi: 10.1007/s12264-013-1358-3. Epub 2013 Jul 16. Neurosci Bull. 2013. PMID: 23861090 Free PMC article. Review.
References
-
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr, Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. - PubMed
-
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

