Spatial updating and the maintenance of visual constancy
- PMID: 18786618
- PMCID: PMC2677727
- DOI: 10.1016/j.neuroscience.2008.07.079
Spatial updating and the maintenance of visual constancy
Abstract
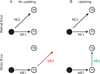
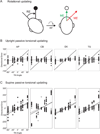
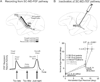
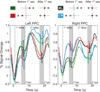
Spatial updating is the means by which we keep track of the locations of objects in space even as we move. Four decades of research have shown that humans and non-human primates can take the amplitude and direction of intervening movements into account, including saccades (both head-fixed and head-free), pursuit, whole-body rotations and translations. At the neuronal level, spatial updating is thought to be maintained by receptive field locations that shift with changes in gaze, and evidence for such shifts has been shown in several cortical areas. These regions receive information about the intervening movement from several sources including motor efference copies when a voluntary movement is made and vestibular/somatosensory signals when the body is in motion. Many of these updating signals arise from brainstem regions that monitor our ongoing movements and subsequently transmit this information to the cortex via pathways that likely include the thalamus. Several issues of debate include (1) the relative contribution of extra-retinal sensory and efference copy signals to spatial updating, (2) the source of an updating signal for real life, three-dimensional motion that cannot arise from brain areas encoding only two-dimensional commands, and (3) the reference frames used by the brain to integrate updating signals from various sources. This review highlights the relevant spatial updating studies and provides a summary of the field today. We find that spatial constancy is maintained by a highly evolved neural mechanism that keeps track of our movements, transmits this information to relevant brain regions, and then uses this information to change the way in which single neurons respond. In this way, we are able to keep track of relevant objects in the outside world and interact with them in meaningful ways.
Figures







Similar articles
-
Roles of gravitational cues and efference copy signals in the rotational updating of memory saccades.J Neurophysiol. 2005 Jul;94(1):468-78. doi: 10.1152/jn.00700.2004. Epub 2005 Feb 16. J Neurophysiol. 2005. PMID: 15716372
-
Human visuospatial updating after passive translations in three-dimensional space.J Neurophysiol. 2008 Apr;99(4):1799-809. doi: 10.1152/jn.01091.2007. Epub 2008 Feb 6. J Neurophysiol. 2008. PMID: 18256164 Free PMC article.
-
Updating visual memory across eye movements for ocular and arm motor control.J Neurophysiol. 2008 Nov;100(5):2507-14. doi: 10.1152/jn.90599.2008. Epub 2008 Sep 3. J Neurophysiol. 2008. PMID: 18768640
-
Spatial constancy mechanisms in motor control.Philos Trans R Soc Lond B Biol Sci. 2011 Feb 27;366(1564):476-91. doi: 10.1098/rstb.2010.0089. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21242137 Free PMC article. Review.
-
Neural control of 3-D gaze shifts in the primate.Prog Brain Res. 2003;142:109-24. doi: 10.1016/s0079-6123(03)42009-8. Prog Brain Res. 2003. PMID: 12693257 Review.
Cited by
-
Role of Alpha-Band Oscillations in Spatial Updating across Whole Body Motion.Front Psychol. 2016 May 6;7:671. doi: 10.3389/fpsyg.2016.00671. eCollection 2016. Front Psychol. 2016. PMID: 27199882 Free PMC article.
-
On the Dynamics of Spatial Updating.Front Neurosci. 2022 Feb 17;16:780027. doi: 10.3389/fnins.2022.780027. eCollection 2022. Front Neurosci. 2022. PMID: 35250442 Free PMC article.
-
Role of Rostral Fastigial Neurons in Encoding a Body-Centered Representation of Translation in Three Dimensions.J Neurosci. 2018 Apr 4;38(14):3584-3602. doi: 10.1523/JNEUROSCI.2116-17.2018. Epub 2018 Feb 27. J Neurosci. 2018. PMID: 29487123 Free PMC article.
-
Spatiotopic coding during dynamic head tilt.J Neurophysiol. 2017 Feb 1;117(2):808-817. doi: 10.1152/jn.00508.2016. Epub 2016 Nov 30. J Neurophysiol. 2017. PMID: 27903636 Free PMC article.
-
Avoidance of a moving threat in the common chameleon (Chamaeleo chamaeleon): rapid tracking by body motion and eye use.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016 Aug;202(8):567-76. doi: 10.1007/s00359-016-1106-z. Epub 2016 Jun 24. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2016. PMID: 27343128
References
-
- Admiraal MA, Keijsers NL, Gielen CC. Gaze affects pointing toward remembered visual targets after a self-initiated step. J Neurophysiol. 2004;92:2380–2393. - PubMed
-
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. - PubMed
-
- Aubert H. Eine scheinbare bedeutende Drehung von Objekten bei Neigung des Kopfes nach rechts oder links. Virchows Arch. 1861;20:381–393.
-
- Avillac M, Deneve S, Olilvier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neuro. 2005;8:941–949. - PubMed
-
- Baker JT, Harper TM, Snyder LH. Spatial memory following shifts of gaze. I. Saccades to memorized world-fixed and gaze-fixed targets. J Neurophysiol. 2003;89:2564–2576. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

