Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation
- PMID: 18786784
- PMCID: PMC3896447
- DOI: 10.1016/j.ijrobp.2008.04.071
Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation
Abstract
Purpose: Total-body irradiation (TBI) has an important role in patients undergoing hematopoietic cell transplantation (HCT), but is associated with significant toxicities. Targeted TBI using helical tomotherapy results in reduced doses to normal organs, which predicts for reduced toxicities compared with standard TBI.
Methods and materials: Thirteen patients with multiple myeloma were treated in an autologous tandem transplantation Phase I trial with high-dose melphalan, followed 6 weeks later by total-marrow irradiation (TMI) to skeletal bone. Dose levels were 10, 12, 14, and 16 Gy at 2 Gy daily/twice daily. In a separate allogeneic HCT trial, 8 patients (5 with acute myelogenous leukemia, 1 with acute lymphoblastic leukemia, 1 with non-Hodgkin's lymphoma, and 1 with multiple myeloma) were treated with TMI plus total lymphoid irradiation plus splenic radiotherapy to 12 Gy (1.5 Gy twice daily) combined with fludarabine/melphalan.
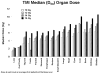
Results: For the 13 patients in the tandem autologous HCT trial, median age was 54 years (range, 42-66 years). Median organ doses were 15-65% that of the gross target volume dose. Primarily Grades 1-2 acute toxicities were observed. Six patients reported no vomiting; 9 patients, no mucositis; 6 patients, no fatigue; and 8 patients, no diarrhea. For the 8 patients in the allogeneic HCT trial, median age was 52 years (range, 24-61 years). Grades 2-3 nausea, vomiting, mucositis, and diarrhea were observed. In both trials, no Grade 4 nonhematologic toxicity was observed, and all patients underwent successful engraftment.
Conclusions: This study shows that TMI using helical tomotherapy is clinically feasible. The reduced acute toxicities observed compare favorably with those seen with standard TBI. Initial results are encouraging and warrant further evaluation as a method to dose escalate with acceptable toxicity or to offer TBI-containing regimens to patients unable to tolerate standard approaches.
Conflict of interest statement
Conflict of Interest: Jeffrey Y.C. Wong, M.D. has previously received honoraria from TomoTherapy, Inc. for speaking engagements.
Figures

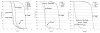
Similar articles
-
Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: an alternative to standard total body irradiation.Biol Blood Marrow Transplant. 2006 Mar;12(3):306-15. doi: 10.1016/j.bbmt.2005.10.026. Biol Blood Marrow Transplant. 2006. PMID: 16503500
-
Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation.Int J Radiat Oncol Biol Phys. 2013 Jan 1;85(1):148-56. doi: 10.1016/j.ijrobp.2012.03.033. Epub 2012 May 15. Int J Radiat Oncol Biol Phys. 2013. PMID: 22592050 Free PMC article. Clinical Trial.
-
Phase 1 Study of the Combination of Escalated Total Marrow Irradiation Using Helical Tomotherapy and Fixed High-Dose Melphalan (140 mg/m²) Followed by Autologous Stem Cell Transplantation at First Relapse in Multiple Myeloma.Int J Radiat Oncol Biol Phys. 2023 Mar 1;115(3):677-685. doi: 10.1016/j.ijrobp.2022.09.069. Epub 2022 Sep 27. Int J Radiat Oncol Biol Phys. 2023. PMID: 36174802 Clinical Trial.
-
Total marrow irradiation (TMI): Addressing an unmet need in hematopoietic cell transplantation - a single institution experience review.Front Oncol. 2022 Oct 3;12:1003908. doi: 10.3389/fonc.2022.1003908. eCollection 2022. Front Oncol. 2022. PMID: 36263219 Free PMC article. Review.
-
Rationale, implementation considerations, delineation and planning target objective recommendations for volumetric modulated arc therapy and helical tomotherapy total body irradiation, total marrow irradiation, total marrow and lymphoid irradiation and total lymphoid irradiation.Radiother Oncol. 2025 May;206:110822. doi: 10.1016/j.radonc.2025.110822. Epub 2025 Feb 22. Radiother Oncol. 2025. PMID: 39993603 Review.
Cited by
-
Anatomy driven optimization strategy for total marrow irradiation with a volumetric modulated arc therapy technique.J Appl Clin Med Phys. 2012 Jan 5;13(1):3653. doi: 10.1120/jacmp.v13i1.3653. J Appl Clin Med Phys. 2012. PMID: 22231216 Free PMC article.
-
Total marrow lymphoid irradiation IMRT treatment using a novel CT-linac.Eur J Med Res. 2023 Oct 27;28(1):463. doi: 10.1186/s40001-023-01380-4. Eur J Med Res. 2023. PMID: 37884978 Free PMC article.
-
Multi-institutional feasibility study of a fast patient localization method in total marrow irradiation with helical tomotherapy: a global health initiative by the international consortium of total marrow irradiation.Int J Radiat Oncol Biol Phys. 2015 Jan 1;91(1):30-8. doi: 10.1016/j.ijrobp.2014.09.014. Epub 2014 Oct 30. Int J Radiat Oncol Biol Phys. 2015. PMID: 25442340 Free PMC article.
-
Volumetric modulated arc therapy for total body irradiation: A feasibility study using Pinnacle3 treatment planning system and Elekta Agility™ linac.J Appl Clin Med Phys. 2018 Mar;19(2):103-110. doi: 10.1002/acm2.12257. Epub 2018 Jan 24. J Appl Clin Med Phys. 2018. PMID: 29368389 Free PMC article.
-
Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia.Biol Blood Marrow Transplant. 2017 Apr;23(4):618-624. doi: 10.1016/j.bbmt.2017.01.067. Epub 2017 Jan 10. Biol Blood Marrow Transplant. 2017. PMID: 28087456 Free PMC article. Clinical Trial.
References
-
- Blaise D, Maraninchi D, Michallet M, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97:3669–3671. - PubMed
-
- Dusenbery KE, Daniels KA, McClure JS, et al. Randomized comparison of cyclophosphamide-total body irradiation versus busulfan-cyclophosphamide conditioning in autologous bone marrow transplantation for acute myeloid leukemia. Int J Radiation Oncology Biol Phys. 1995;31:119–128. - PubMed
-
- Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: A report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–2730. - PubMed
-
- Bunin N, Aplenc R, Kamani N, et al. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32:543–548. - PubMed
-
- Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: A randomized trial of two irradiation regimens. Blood. 1991;77:1660–1665. - PubMed

