Structure, dynamics and function of nuclear pore complexes
- PMID: 18786826
- PMCID: PMC2697926
- DOI: 10.1016/j.tcb.2008.07.009
Structure, dynamics and function of nuclear pore complexes
Abstract
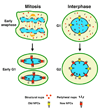
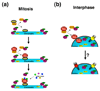
Nuclear pore complexes are large aqueous channels that penetrate the nuclear envelope, thereby connecting the nuclear interior with the cytoplasm. Until recently, these macromolecular complexes were viewed as static structures, the only function of which was to control the molecular trafficking between the two compartments. It has now become evident that this simplistic scenario is inaccurate and that nuclear pore complexes are highly dynamic multiprotein assemblies involved in diverse cellular processes ranging from the organization of the cytoskeleton to gene expression. In this review, we discuss the most recent developments in the nuclear-pore-complex field, focusing on the assembly, disassembly, maintenance and function of this macromolecular structure.
Figures

References
-
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem. 2007;76:647–671. - PubMed
-
- Mosammaparast N, Pemberton LF. Karyopherins: from nuclear-transport mediators to nuclear-function regulators. Trends Cell Biol. 2004;14:547–556. - PubMed
-
- Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

