Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis
- PMID: 18786918
- PMCID: PMC2576536
- DOI: 10.1074/jbc.M805216200
Homomeric and heteromeric assembly of KCNQ (Kv7) K+ channels assayed by total internal reflection fluorescence/fluorescence resonance energy transfer and patch clamp analysis
Abstract
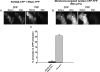
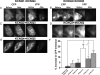
M-type K(+) channels, consisting of KCNQ1-5 (Kv7.1-7.5) subunits, form a variety of homomeric and heteromeric channels. Whereas all the subunits can assemble into homomeric channels, the ability of the subunits to assemble into heteromultimers is highly variable. KCNQ3 is widely thought to co-assemble with several other KCNQ subtypes, whereas KCNQ1 and KCNQ2 do not. However, the existence of other subunit assemblies is not well studied. To systematically explore the heteromeric assembly of KCNQ channels in individual living cells, we performed fluorescence resonance energy transfer (FRET) between cyan fluorescent protein- and yellow fluorescent protein-tagged KCNQ subunits expressed in Chinese hamster ovary cells under total internal reflection fluorescence microscopy in which excitation light only penetrates several hundred nanometers into the cell, thus isolating membrane events. We found significant FRET between homomeric subunits as expected from their functional expression in heterologous expression systems. Also as expected from previous work, robust FRET was observed between KCNQ2 and KCNQ3. KCNQ3 and KCNQ4 also showed substantial FRET as did KCNQ4 and KCNQ5. To determine functional assembly of KCNQ4/KCNQ5 heteromers, we performed two types of experiments. In the first, we constructed a mutant tetraethylammonium ion-sensitive KCNQ4 subunit and tested its assembly with KCNQ5 by patch clamp analysis of the tetraethylammonium ion sensitivity of the resulting current; however, those data were not conclusive. In the second, we co-expressed a KCNQ4 (G285S) pore mutant with KCNQ5 and found the former to act as a dominant negative, suggesting co-assembly of the two types of subunits. These data confirm that among the allowed assembly conformations are KCNQ3/4 and KCNQ4/5 heteromers.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

