Partially irreversible inactivation of mitochondrial aldehyde dehydrogenase by nitroglycerin
- PMID: 18786921
- PMCID: PMC2576553
- DOI: 10.1074/jbc.M804001200
Partially irreversible inactivation of mitochondrial aldehyde dehydrogenase by nitroglycerin
Abstract
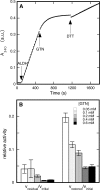
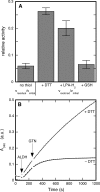
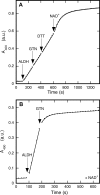
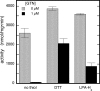
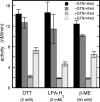
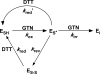
Mitochondrial aldehyde dehydrogenase (ALDH2) may be involved in the biotransformation of glyceryl trinitrate (GTN), and the inactivation of ALDH2 by GTN may contribute to the phenomenon of nitrate tolerance. We studied the GTN-induced inactivation of ALDH2 by UV/visible absorption spectroscopy. Dehydrogenation of acetaldehyde and hydrolysis of p-nitrophenylacetate (p-NPA) were both inhibited by GTN. The rate of inhibition increased with the GTN concentration and decreased with the substrate concentration, indicative of competition between GTN and the substrates. Inactivation of p-NPA hydrolysis was greatly enhanced in the presence of NAD(+), and, to a lesser extent, in the presence of NADH. In the presence of dithiothreitol (DTT) inactivation of ALDH2 was much slower. Dihydrolipoic acid (LPA-H(2)) was less effective than DTT, whereas glutathione, cysteine, and ascorbate did not protect against inactivation. When DTT was added after complete inactivation, dehydrogenase reactivation was quite modest (< or =16%). The restored dehydrogenase activity correlated inversely with the GTN concentration but was hardly affected by the concentrations of acetaldehyde or DTT. Partial reactivation of dehydrogenation was also accomplished by LPA-H(2) but not by GSH. We conclude that, in addition to the previously documented reversible inhibition by GTN that can be ascribed to the oxidation of the active site thiol, there is an irreversible component to ALDH inactivation. Importantly, ALDH2-catalyzed GTN reduction was partly inactivated by preincubation with GTN, suggesting that the inactivation of GTN reduction is also partly irreversible. These observations are consistent with a significant role for irreversible inactivation of ALDH2 in the development of nitrate tolerance.
Figures



Similar articles
-
Characterization of the East Asian variant of aldehyde dehydrogenase-2: bioactivation of nitroglycerin and effects of Alda-1.J Biol Chem. 2010 Jan 8;285(2):943-52. doi: 10.1074/jbc.M109.014548. Epub 2009 Nov 11. J Biol Chem. 2010. PMID: 19906643 Free PMC article.
-
Different effects of ascorbate deprivation and classical vascular nitrate tolerance on aldehyde dehydrogenase-catalysed bioactivation of nitroglycerin.Br J Pharmacol. 2009 Apr;156(8):1248-55. doi: 10.1111/j.1476-5381.2009.00126.x. Epub 2009 Feb 27. Br J Pharmacol. 2009. PMID: 19254277 Free PMC article.
-
Site-directed mutagenesis of aldehyde dehydrogenase-2 suggests three distinct pathways of nitroglycerin biotransformation.Mol Pharmacol. 2011 Aug;80(2):258-66. doi: 10.1124/mol.111.071704. Epub 2011 May 2. Mol Pharmacol. 2011. PMID: 21536753
-
Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase.Trends Cardiovasc Med. 2006 Nov;16(8):259-65. doi: 10.1016/j.tcm.2006.05.001. Trends Cardiovasc Med. 2006. PMID: 17055381 Review.
-
Nitroglycerin use in myocardial infarction patients.Circ J. 2012;76(1):15-21. doi: 10.1253/circj.cj-11-1133. Epub 2011 Nov 1. Circ J. 2012. PMID: 22040938 Free PMC article. Review.
Cited by
-
Organic nitrate metabolism and action: toward a unifying hypothesis and the future-a dedication to Professor Leslie Z. Benet.J Pharm Sci. 2013 Sep;102(9):3070-81. doi: 10.1002/jps.23550. Epub 2013 May 13. J Pharm Sci. 2013. PMID: 23670666 Free PMC article. Review.
-
Aldehyde dehydrogenase 2 in cardiac protection: a new therapeutic target?Trends Cardiovasc Med. 2009 Jul;19(5):158-64. doi: 10.1016/j.tcm.2009.09.003. Trends Cardiovasc Med. 2009. PMID: 20005475 Free PMC article. Review.
-
Changes in aldehyde dehydrogenase 2 expression in rat blood vessels during glyceryl trinitrate tolerance development and reversal.Br J Pharmacol. 2011 Sep;164(2b):632-43. doi: 10.1111/j.1476-5381.2011.01448.x. Br J Pharmacol. 2011. PMID: 21506955 Free PMC article.
-
Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress.Antioxid Redox Signal. 2015 Oct 10;23(11):899-942. doi: 10.1089/ars.2015.6376. Epub 2015 Sep 24. Antioxid Redox Signal. 2015. PMID: 26261901 Free PMC article. Review.
-
Advances in Factors Affecting ALDH2 Activity and its Mechanisms.Cardiovasc Toxicol. 2024 Dec;24(12):1428-1438. doi: 10.1007/s12012-024-09923-9. Epub 2024 Oct 4. Cardiovasc Toxicol. 2024. PMID: 39365551 Review.
References
-
- Mayer, B. (2003) Angew. Chem. Int. Ed. 42 388-391 - PubMed
-
- Chen, Z., and Stamler, J. S. (2006) Trends Cardiovasc. Med. 16 259-265 - PubMed
-
- Feelisch, M. (1998) Naunyn-Schmiedeberg's Arch. Pharmacol. 358 113-122 - PubMed
-
- Wang, P. G., Xian, M., Tang, X., Wu, X., Wen, Z., Cai, T., and Janczuk, A. J. (2002) Chem. Rev. 102 1091-1134 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

