Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation
- PMID: 18786924
- PMCID: PMC2581578
- DOI: 10.1074/jbc.M802814200
Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation
Abstract
Interleukin-18 (IL-18), a pro-inflammatory cytokine, is a key factor in inflammatory bowel disease (IBD). Caspase-1 activates this cytokine, but other proteases are likely involved in maturation. Because meprin metalloproteinases have been implicated in IBD, the interaction of these proteases with proIL-18 was studied. The results demonstrate that the meprin beta subunit of meprins A and B cleaves proIL-18 into a smaller 17-kDa product. The cleavage is at the Asn51-Asp52 bond, a site C-terminal to caspase-1 cleavage. The cleavage occurred in vitro with a Km of 1.3 microm and in Madin-Darby canine kidney cells transfected with meprin beta when proIL-18 was added to the culture medium. The product of meprin B cleavage of proIL-18 activated NF-kappaB in EL-4 cells, indicating that it was biologically active. To determine the physiological significance of the interactions of meprins with proIL-18, an experimental model of IBD was produced by administering dextran sulfate sodium (DSS) to wild-type and meprin beta knock-out (betaKO) mice, and the serum levels of active IL-18 were determined. DSS-treated meprin betaKO mice had lower levels of the active cytokine in the serum compared with wild-type mice. Furthermore, in meprin alphaKO mice, which express meprin beta but not alpha, active IL-18 was elevated in the serum of DSS-treated mice compared with wild-type mice, indicating that the meprin isoforms have opposing effects on the IL-18 levels in vivo. This study identifies proIL-18 as a biologically important substrate for meprin beta and implicates meprins in the modulation of inflammation.
Figures

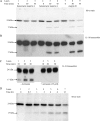


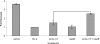

References
-
- Fantuzzi, G., and Dinarello, C. A. (1999) J. Clin. Immunol. 19 1–11 - PubMed
-
- Reuter, B. K., and Pizarro, T. T. (2004) Eur. J. Immunol. 34 2347–2355 - PubMed
-
- Mehta, V. B., Hart, J., and Wewers, M. D. (2001) J. Biol. Chem. 276 3820–3826 - PubMed
-
- Bond, J. S., Matters, G. L., Banerjee, S., and Dusheck, R. E. (2005) FEBS Lett. 579 3317–3322 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

