Structural interactions between transmembrane helices 4 and 5 of subunit a and the subunit c ring of Escherichia coli ATP synthase
- PMID: 18786930
- PMCID: PMC2581582
- DOI: 10.1074/jbc.M803848200
Structural interactions between transmembrane helices 4 and 5 of subunit a and the subunit c ring of Escherichia coli ATP synthase
Abstract
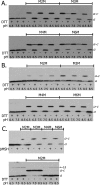
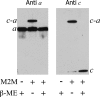
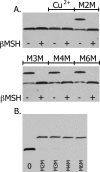
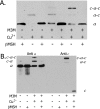
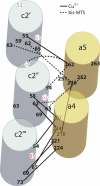
Subunit a plays a key role in promoting H+ transport and the coupled rotary motion of the subunit c ring in F1F0-ATP synthase. H+ binding and release occur at Asp-61 in the middle of the second transmembrane helix (TMH) of F0 subunit c. H+ are thought to reach Asp-61 via aqueous pathways mapping to the surfaces of TMHs 2-5 of subunit a. TMH4 of subunit a is thought to pack close to TMH2 of subunit c based upon disulfide cross-link formation between Cys substitutions in both TMHs. Here we substituted Cys into the fifth TMH of subunit a and the second TMH of subunit c and tested for cross-linking using bis-methanethiosulfonate (bis-MTS) reagents. A total of 62 Cys pairs were tested and 12 positive cross-links were identified with variable alkyl length linkers. Cross-linking was achieved near the middle of the bilayer for the Cys pairs a248C/c62C, a248C/ c63C, a248C/c65C, a251C/c57C, a251C/c59C, a251C/c62C, a252C/c62C, and a252C/c65C. Cross-linking was achieved near the cytoplasmic side of the bilayer for Cys pairs a262C/c53C, a262C/c54C, a262C/c55C, and a263C/c54C. We conclude that both aTMH4 and aTMH5 pack proximately to cTMH2 of the c-ring. In other experiments we demonstrate that aTMH4 and aTMH5 can be simultaneously cross-linked to different subunit c monomers in the c-ring. Five mutants showed pH-dependent cross-linking consistent with aTMH5 changing conformation at lower pH values to facilitate cross-linking. We suggest that the pH-dependent conformational change may be related to the proposed role of aTMH5 in gating H+ access from the periplasm to the cAsp-61 residue in cTMH2.
Figures




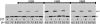
Similar articles
-
The cytoplasmic loops of subunit a of Escherichia coli ATP synthase may participate in the proton translocating mechanism.J Biol Chem. 2008 May 9;283(19):13044-52. doi: 10.1074/jbc.M800900200. Epub 2008 Mar 12. J Biol Chem. 2008. PMID: 18337242 Free PMC article.
-
Obstruction of transmembrane helical movements in subunit a blocks proton pumping by F1Fo ATP synthase.J Biol Chem. 2013 Aug 30;288(35):25535-25541. doi: 10.1074/jbc.M113.496794. Epub 2013 Jul 17. J Biol Chem. 2013. PMID: 23864659 Free PMC article.
-
Aqueous access pathways in subunit a of rotary ATP synthase extend to both sides of the membrane.Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13179-83. doi: 10.1073/pnas.2234364100. Epub 2003 Oct 31. Proc Natl Acad Sci U S A. 2003. PMID: 14595019 Free PMC article.
-
Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ.Biochim Biophys Acta. 2002 Oct 11;1565(2):232-45. doi: 10.1016/s0005-2736(02)00572-2. Biochim Biophys Acta. 2002. PMID: 12409198 Review.
-
Mechanics of coupling proton movements to c-ring rotation in ATP synthase.FEBS Lett. 2003 Nov 27;555(1):29-34. doi: 10.1016/s0014-5793(03)01101-3. FEBS Lett. 2003. PMID: 14630314 Review.
Cited by
-
Fo-driven Rotation in the ATP Synthase Direction against the Force of F1 ATPase in the FoF1 ATP Synthase.J Biol Chem. 2015 Apr 24;290(17):10717-28. doi: 10.1074/jbc.M115.646430. Epub 2015 Feb 24. J Biol Chem. 2015. PMID: 25713065 Free PMC article.
-
F1F0-ATP synthases of alkaliphilic bacteria: lessons from their adaptations.Biochim Biophys Acta. 2010 Aug;1797(8):1362-77. doi: 10.1016/j.bbabio.2010.02.028. Epub 2010 Mar 1. Biochim Biophys Acta. 2010. PMID: 20193659 Free PMC article. Review.
-
Structure and mechanism of the ATP synthase membrane motor inferred from quantitative integrative modeling.J Gen Physiol. 2016 Dec;148(6):441-457. doi: 10.1085/jgp.201611679. Epub 2016 Nov 7. J Gen Physiol. 2016. PMID: 27821609 Free PMC article.
-
Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane.J Biol Chem. 2010 Dec 17;285(51):39811-8. doi: 10.1074/jbc.M110.175844. Epub 2010 Oct 13. J Biol Chem. 2010. PMID: 20943664 Free PMC article.
-
Structural study on the architecture of the bacterial ATP synthase Fo motor.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):E2050-6. doi: 10.1073/pnas.1203971109. Epub 2012 Jun 26. Proc Natl Acad Sci U S A. 2012. PMID: 22736796 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

