Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling
- PMID: 18786989
- PMCID: PMC2573246
- DOI: 10.1128/JVI.01646-08
Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling
Abstract
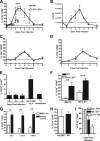
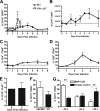
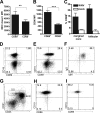
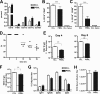
The B-cell response against West Nile virus (WNV), an encephalitic Flavivirus of global concern, is critical to controlling central nervous system dissemination and neurological sequelae, including death. Here, using a well-characterized mouse model of WNV infection, we examine the factors that govern early B-cell activation. Subcutaneous inoculation with a low dose of replicating WNV results in extensive B-cell activation in the draining lymph node (LN) within days of infection as judged by upregulation of the surface markers CD69, class II major histocompatibility complex, and CD86 on CD19(+) cells. B-cell activation in the LN but not the spleen was dependent on signals through the type I alpha/beta interferon (IFN-alpha/beta) receptor. Despite significant activation in the draining LN at day 3 after infection, WNV-specific B cells were not detected by immunoglobulin M enzyme-linked immunospot analysis until day 7. Liposome depletion experiments demonstrate that B-cell activation after WNV infection was not affected by the loss of F4/80(+) or CD169(+) subcapsular macrophages. Nonetheless, LN myeloid cells were essential for control of viral replication and survival from infection. Overall, our data suggest that the massive, early polyclonal B-cell activation occurring in the draining LN after WNV infection is immunoglobulin receptor and macrophage independent but requires sustained signals through the type I IFN-alpha/beta receptor.
Figures
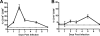

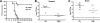
References
-
- Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9143-150. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413732-738. - PubMed
-
- Alsharifi, M., M. Lobigs, M. Regner, E. Lee, A. Koskinen, and A. Mullbacher. 2005. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J. Immunol. 1754635-4640. - PubMed
-
- Ben-Nathan, D., I. Huitinga, S. Lustig, N. van Rooijen, and D. Kobiler. 1996. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch. Virol. 141459-469. - PubMed
-
- Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 2982199-2202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

