Role of cellular glycosaminoglycans and charged regions of viral G protein in human metapneumovirus infection
- PMID: 18786997
- PMCID: PMC2583676
- DOI: 10.1128/JVI.01208-08
Role of cellular glycosaminoglycans and charged regions of viral G protein in human metapneumovirus infection
Abstract
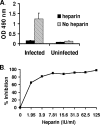
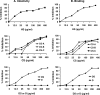
Human metapneumovirus (hMPV) is an important cause of lower respiratory tract disease, particularly in infants and young children. hMPV has two major glycoproteins, G and F, which are responsible for virus attachment and membrane fusion, respectively. We investigated the role of cellular glycosaminoglycans (GAGs) and G protein in hMPV infection. The pretreatment of hMPV with soluble heparin markedly inhibited the infection of HEp-2 cells. Recombinant G protein, comprising the extracellular domain of G, bound to heparin-agarose columns and also to HEp-2 cells. hMPV infection and G protein binding to HEp-2 cells was inhibited by other soluble GAGs, including chondroitin sulfates, by the enzymatic removal of cell surface GAGs with GAG lyases or by the pretreatment of cells with basic fibroblast growth factor. The role of cellular GAGs was confirmed by the binding of G protein to wild-type CHO cells but not to GAG-deficient CHO-pgsA745 cells. An analysis of the G protein sequence revealed two adjacent clusters of positively charged amino acids ((149)EKKKTRA(155) and (159)QRRGKGKE(166)). Truncated G fragments were expressed, and only the fragment containing these putative heparin binding domains retained heparin binding. The alanine mutagenesis of charged residues in either of these regions resulted in the loss of binding to heparin and to HEp-2 cells, suggesting that both sites are likely to be required for hMPV attachment. These results, taken together with the inhibition of hMPV infection by soluble G protein, indicate an important role for G protein and cellular GAGs in hMPV infection.
Figures





Similar articles
-
DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans.J Virol. 2016 Aug 12;90(17):7848-63. doi: 10.1128/JVI.00537-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334579 Free PMC article.
-
Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection.Virology. 2000 Jun 5;271(2):264-75. doi: 10.1006/viro.2000.0293. Virology. 2000. PMID: 10860881
-
Diversity in glycosaminoglycan binding amongst hMPV G protein lineages.Viruses. 2012 Dec 14;4(12):3785-803. doi: 10.3390/v4123785. Viruses. 2012. PMID: 23242371 Free PMC article.
-
Breaking in: human metapneumovirus fusion and entry.Viruses. 2013 Jan 16;5(1):192-210. doi: 10.3390/v5010192. Viruses. 2013. PMID: 23325326 Free PMC article. Review.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
Cited by
-
Evidence for the interaction of the human metapneumovirus G and F proteins during virus-like particle formation.Virol J. 2013 Sep 25;10:294. doi: 10.1186/1743-422X-10-294. Virol J. 2013. PMID: 24067107 Free PMC article.
-
Immunological insights into the re-emergence of human metapneumovirus.Curr Opin Immunol. 2025 Jun;94:102562. doi: 10.1016/j.coi.2025.102562. Epub 2025 May 12. Curr Opin Immunol. 2025. PMID: 40359650 Review.
-
DC-SIGN and L-SIGN Are Attachment Factors That Promote Infection of Target Cells by Human Metapneumovirus in the Presence or Absence of Cellular Glycosaminoglycans.J Virol. 2016 Aug 12;90(17):7848-63. doi: 10.1128/JVI.00537-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334579 Free PMC article.
-
Novel insights into the host cell glycan binding profile of human metapneumovirus.J Virol. 2024 Jun 13;98(6):e0164123. doi: 10.1128/jvi.01641-23. Epub 2024 May 1. J Virol. 2024. PMID: 38690874 Free PMC article.
-
A Re-emerging Respiratory Virus: Human Metapneumovirus (hMPV).Cureus. 2025 Feb 1;17(2):e78354. doi: 10.7759/cureus.78354. eCollection 2025 Feb. Cureus. 2025. PMID: 40034641 Free PMC article. Review.
References
-
- Baba, M., R. Snoeck, R. Pauwels, and E. de Clercq. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 321742-1745. - PMC - PubMed
-
- Biacchesi, S., Q. N. Pham, M. H. Skiadopoulos, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2005. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J. Virol. 7912608-12613. - PMC - PubMed
-
- Biacchesi, S., M. H. Skiadopoulos, G. Boivin, C. T. Hanson, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2003. Genetic diversity between human metapneumovirus subgroups. Virology 3151-9. - PubMed
-
- Biacchesi, S., M. H. Skiadopoulos, L. Yang, E. W. Lamirande, K. C. Tran, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2004. Recombinant human metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J. Virol. 7812877-12887. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

