With minimal systemic T-cell expansion, CD8+ T Cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus
- PMID: 18787003
- PMCID: PMC2573271
- DOI: 10.1128/JVI.01433-08
With minimal systemic T-cell expansion, CD8+ T Cells mediate protection of rhesus macaques immunized with attenuated simian-human immunodeficiency virus SHIV89.6 from vaginal challenge with simian immunodeficiency virus
Abstract
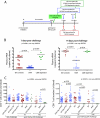
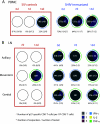
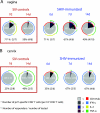
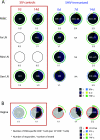
The presence, at the time of challenge, of antiviral effector T cells in the vaginal mucosa of female rhesus macaques immunized with live-attenuated simian-human immunodeficiency virus 89.6 (SHIV89.6) is associated with consistent and reproducible protection from pathogenic simian immunodeficiency virus (SIV) vaginal challenge (18). Here, we definitively demonstrate the protective role of the SIV-specific CD8(+) T-cell response in SHIV-immunized monkeys by CD8(+) lymphocyte depletion, an intervention that abrogated SHIV-mediated control of challenge virus replication and largely eliminated the SIV-specific T-cell responses in blood, lymph nodes, and genital mucosa. While in the T-cell-intact SHIV-immunized animals, polyfunctional and degranulating SIV-specific CD8(+) T cells were present in the genital tract and lymphoid tissues from the day of challenge until day 14 postchallenge, strikingly, expansion of SIV-specific CD8(+) T cells in the immunized monkeys was minimal and limited to the vagina. Thus, protection from uncontrolled SIV replication in animals immunized with attenuated SHIV89.6 is primarily mediated by CD8(+) T cells that do not undergo dramatic systemic expansion after SIV challenge. These findings demonstrate that despite, and perhaps because of, minimal systemic expansion of T cells at the time of challenge, a stable population of effector-cytotoxic CD8(+) T cells can provide significant protection from vaginal SIV challenge.
Figures




Similar articles
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Live-attenuated lentivirus immunization modulates innate immunity and inflammation while protecting rhesus macaques from vaginal simian immunodeficiency virus challenge.J Virol. 2012 Sep;86(17):9188-200. doi: 10.1128/JVI.00532-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696662 Free PMC article.
-
Protective attenuated lentivirus immunization induces SIV-specific T cells in the genital tract of rhesus monkeys.Mucosal Immunol. 2008 May;1(3):219-28. doi: 10.1038/mi.2008.6. Epub 2008 Mar 5. Mucosal Immunol. 2008. PMID: 19079181 Free PMC article.
-
Limited dissemination of pathogenic SIV after vaginal challenge of rhesus monkeys immunized with a live, attenuated lentivirus.Virology. 2009 Sep 30;392(2):260-70. doi: 10.1016/j.virol.2009.06.052. Epub 2009 Aug 3. Virology. 2009. PMID: 19647847 Free PMC article.
-
Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6.J Med Primatol. 2005 Oct;34(5-6):271-81. doi: 10.1111/j.1600-0684.2005.00125.x. J Med Primatol. 2005. PMID: 16128922 Free PMC article. Review.
Cited by
-
Gag p27-specific B- and T-cell responses in Simian immunodeficiency virus SIVagm-infected African green monkeys.J Virol. 2009 Mar;83(6):2770-7. doi: 10.1128/JVI.01841-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109377 Free PMC article.
-
Localized populations of CD8 MHC class I tetramer SIV-specific T cells in lymphoid follicles and genital epithelium.PLoS One. 2009;4(1):e4131. doi: 10.1371/journal.pone.0004131. Epub 2009 Jan 5. PLoS One. 2009. PMID: 19122815 Free PMC article.
-
Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens.J Immunol. 2009 May 15;182(10):6587-99. doi: 10.4049/jimmunol.0900317. J Immunol. 2009. PMID: 19414814 Free PMC article.
-
Extralymphoid CD8+ T cells resident in tissue from simian immunodeficiency virus SIVmac239{Delta}nef-vaccinated macaques suppress SIVmac239 replication ex vivo.J Virol. 2010 Apr;84(7):3362-72. doi: 10.1128/JVI.02028-09. Epub 2010 Jan 20. J Virol. 2010. PMID: 20089651 Free PMC article.
-
Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine.J Virol. 2012 Feb;86(4):2239-50. doi: 10.1128/JVI.06175-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156519 Free PMC article. Clinical Trial.
References
-
- Abdel-Motal, U. M., J. Gillis, K. Manson, M. Wyand, D. Montefiori, K. Stefano-Cole, R. C. Montelaro, J. D. Altman, and R. P. Johnson. 2005. Kinetics of expansion of SIV Gag-specific CD8+ T lymphocytes following challenge of vaccinated macaques. Virology 333226-238. - PubMed
-
- Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 773099-3118. - PMC - PubMed
-
- Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 1606062-6071. - PubMed
-
- Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. - PMC - PubMed
-
- Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 19263-75. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

